Globally, breast cancer is the most frequently occurring cancer as well as the common cause of cancer death in women, with an estimated 1.7 million cases and 521 900 deaths in 2012. It accounts for 25.2% of all incidents cancers, and 14.7% of the cancer deaths among women worldwide.1 Globally, there has been a sharp rise in breast cancer cases. From 2008 to 2012, the incidence of breast cancer increased by more than 20%, while mortality increased by 14%.2 In many developing countries, the incidence of breast cancer is rising sharply due to changes in reproductive factors, lifestyle, and increased life expectancy.3,4
In terms of mortality, and according to GLOBOCAN 2012 estimates, breast cancer is the second cause of cancer death in more developed regions while it is the most frequent cause of cancer death in women in less developed regions.1 Although the incidence rate of breast cancer is lower in developing countries than in most developed countries, case fatality rates are very high. This high case fatality rate is likely due to lack of awareness of the importance of early detection and treatment, and the scarcity of adequate facilities for detection and diagnosis as well as poor access to primary treatment.5
In Iraq during 2015, 4824 cases of breast cancer were reported accounting for 19.1% of all newly diagnosed cancers and 33.5% of the registered female cancers, with an incidence rate of 25.8 per 100 000 female population.6 In Basrah, breast cancer represents a significant health problem. It ranked as number one cancer among women accounting for 25.0% of all reported cases,7 and the current crude incidence rate is 23.7/100 000.8 In 2005, breast cancer accounted for 10.8% of cancer-related deaths in Basrah with a cause-specific death rate of 3.2/100 000 population.9
In recent decades, breast cancer incidence and survival have changed considerably in many countries. In many developed countries, despite the increasing incidence, the survival rates of breast cancer patients were substantially improved.10 In developing countries, women usually present late and are expected to have a limited life span.11
The prognosis and management prediction of breast cancer is affected by several clinical and histopathological factors including stage and grade, histological type, estrogen receptors (ER), progesterone receptors (PR), human epidermal growth factor receptor 2 (HER2/neu), and expression of Ki67. Each of these parameters determines the biology and aggression of tumor alone or in combination. In addition, they can predict the opportunity to treat breast cancer properly.12
According to the expression levels of Ki67 and the status of PR, ER, and HER2/neu, breast cancer was broadly categorized into four molecular subtypes: luminal A, luminal B, HER2/neu overexpression, and triple-negative breast cancer (TNBC). Each type marked a certain aggression and prognosis.13 A Chinese study showed that TNBC has a high risk of relapse and death.14
Lymph node status was also reported as an important prognostic variable for determining outcome in patients with ER+ breast cancer.15 While patients with breast cancer positive lymph node status showed a poor prognosis compared with their negative lymph node status counterparts, some of those patients responded well to treatment and attained a good survival. It is not well known whether lymph node involvement is simply a sign of tumor progression over time or whether a primary tumor’s ability to metastasize is predetermined by its biology.16
The survival rate is an important measure of cancer severity and an essential mean for monitoring and evaluating the effectiveness of cancer control.17 Further, there is no information on breast cancer survival in Basrah. Therefore, we sought to determine the overall observed three-year survival rate of women with breast cancer diagnosed in 2013/2014 in Basrah, and to investigate the determinants of the survival.
Methods
This was a prospective registry-based study done at the Oncology Center in Al-Sadr Teaching Hospital in Basrah. This is the only center in Basrah that delivers management services for adult cancer patients, particularly those with solid tumors.
The study population was women with histologically verified primary breast cancer (ICD-10 classification codes: C50.0–C50.9),18 living in Basrah and newly diagnosed and registered in the aforementioned center between 1 January 2013 and 31 December 2014.
The total number of registered cases during these two years was 605 (299 cases in 2013 and 306 cases in 2014), which represents the sample size of this study. The study sample included only Basrah inhabitant women to ensure accurate follow-up.
Data about sociodemographic characteristics such as age, residence, and occupation, in addition to information related to breast cancer characteristics (histopathology, tumor grade and stage) and treatment were extracted from Basrah Oncology Center registry records and patients’ files according to a special form designed for the study. All medical records of breast cancer patients diagnosed in the period 2013–2014 were identified with the help of medical records administrative staff. In cases of incomplete medical records, additional data were searched via histopathological laboratory of Al-Sadr Teaching Hospital, Cancer Control Department, and the statistics department at Basrah General Health Directorate.
Each patient was followed-up until dead or alive at the end of the data collection period (i.e., for up to three years from the date of diagnosis to the 31 December 2017 cutoff point). Therefore, the only considered outcome in this study is survival. Information about death was gathered from the Basrah Oncology Center registry. In addition, the death registry from the Statistics Department using the patient’s name was used to confirm death for all cases and those who lost contact with the center.
Duplication of data was avoided by using the patient’s full four names (patient, father, grandfather, and family name) in addition to the mother’s name. The data were typed first on an Excel sheet then matched and checked for any repetition both manually and electronically. The data were then transmitted into an SPSS Statistics (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) computer file for statistical analysis.
Tumor morphology was grouped into invasive ductal carcinoma (IDC), invasive lobular carcinoma, and other types of carcinoma (including mucinous, scirrhous, medullary, lymphoma, and variants of IDC).19 Tumor stage was classified into four categories according to the TNM classification of malignant tumors of the 6th edition of the American Joint Committee on Cancer Classification: stage I (T1N0M0), stage II (T0–1N1M0, T2N0–1M0, T3N0M0), stage III (T0–2N2M0, T3N1–2M0, T4N0–2M0, T0–4N3M0), and stage IV (T0–4N0–3M1).20 Tumor grade was classified into three classes (1 to 3), based on the Nottingham grading system.21 Observed survival rate was defined as “the actual percentage of patients still alive at some specified time after diagnosis of cancer. It considers deaths from all causes, cancer or otherwise”.22 The observation time was defined as the time between the date of diagnosis and death. In the absence of an event, the observation time was censored at the end date of follow-up.23
Official approval for the study was obtained from the ethics committee of Basrah General Health Directorate before starting data collection.
Frequencies of sociodemographic and clinical characteristics of all patients were calculated.Survival rates were estimated by the Kaplan-Meier method, and the log-rank test was used to determine the differences in survival time in subgroups. Cox proportional hazard regression analysis was performed to assess the independent effect of various prognostic factors on survival and the risk of each of these factors. The following variables were entered in the regression model: age, morphology, grade, treatment, and stage of breast cancer. A p-value < 0.050 was considered statistically significant.
Table 1: Basic characteristics of the study population.
|
Age, years |
|
|
|
< 35 |
52 |
8.6 |
|
35–44 |
146 |
24.1 |
|
45–54 |
184 |
30.4 |
|
55–64 |
145 |
24.0 |
|
≥ 65 |
78 |
12.9 |
|
Grade |
|
|
|
Well differentiated |
43 |
7.1 |
|
Moderately differentiated |
354 |
58.5 |
|
Poorly differentiated |
208 |
34.4 |
|
Stage |
|
|
|
I |
45 |
7.4 |
|
II |
293 |
48.4 |
|
III |
183 |
30.2 |
|
IV |
84 |
13.9 |
|
Morphology |
|
|
|
Ductal carcinoma |
543 |
89.8 |
|
Lobular |
24 |
4.0 |
|
Others |
38 |
6.3 |
|
Treatment |
|
|
|
Surgery |
77 |
12.7 |
|
Chemotherapy |
30 |
5.0 |
|
Surgery and radiotherapy |
88 |
14.5 |
|
Surgery and chemotherapy |
116 |
19.2 |
|
Surgery, chemotherapy, and radiotherapy |
294 |
48.6 |
Table 2: Survival rates (Kaplan-Meier analysis) of breast cancer cases in women diagnosed in Basrah during 2013–2014.
|
Age, years |
|
|
|
|
|
|
|
< 35 |
52 |
11 (21.2) |
33.3 |
31.5–35.1 |
78.8 |
0.068 |
|
35–44 |
146 |
17 (11.6) |
33.8 |
32.7–34.9 |
88.4 |
|
45–54 |
184 |
25 13.6) |
33.6 |
32.6–34.6 |
86.4 |
|
55–64 |
145 |
32 (22.1) |
31.6 |
30.1–32.2 |
77.9 |
|
≥ 65 |
78 |
16 (20.5) |
30.9 |
28.6–33.4 |
79.5 |
|
Grade |
|
|
|
|
|
|
|
Well differentiated |
43 |
3 (7.0) |
35.2 |
34.3–36.1 |
93.0 |
< 0.001 |
|
Moderately differentiated |
354 |
39 (11.0) |
33.9 |
33.2–34.7 |
89.0 |
|
Poorly differentiated |
208 |
59 (28.4) |
30.5 |
29.0–31.9 |
71.6 |
|
Stage |
|
|
|
|
|
|
|
I |
45 |
1 (2.2) |
35.3 |
33.9–36.6 |
97.8 |
< 0.001 |
|
II |
293 |
27 (9.2) |
34.4 |
33.7–35.1 |
90.8 |
|
III |
183 |
40 (21.9) |
32.3 |
31.1–33.5 |
78.1 |
|
IV |
84 |
33 (39.3) |
27.3 |
24.6–30.0 |
60.7 |
|
Morphology |
|
|
|
|
|
|
|
Ductal |
543 |
92 (16.9) |
32.8 |
32.1–33.5 |
83.1 |
0.528 |
|
Lobular |
24 |
2 (8.3) |
34.4 |
31.7–37.2 |
91.7 |
|
Others |
38 |
7 (18.4) |
32.1 |
29.1–35.1 |
81.6 |
|
Treatment |
|
|
|
|
|
|
|
Surgery |
77 |
10 (13.0) |
33.8 |
32.4–35.2 |
87.0 |
|
Chemotherapy |
30 |
14 (46.7) |
25.5 |
20.8–30.1 |
53.3 |
|
Surgery and radiotherapy |
88 |
12 (13.6) |
34.2 |
32.9–35.4 |
86.4 |
|
Surgery and chemotherapy |
116 |
20 (17.2) |
32.6 |
31.0–34.2 |
82.8 |
CI: confidence interval.
Table 3: Cox proportional hazard regression analysis.
|
Age, years |
|
|
|
|
|
< 35 |
1.00 |
- |
- |
- |
|
35–44 |
1.06 |
0.53 |
2.10 |
0.103 |
|
45–54 |
1.96 |
0.97 |
3.97 |
0.019 |
|
55–64 |
2.58 |
1.17 |
5.65 |
0.050 |
|
≥ 65 |
2.55 |
0.83 |
6.87 |
0.168 |
|
Grade |
|
|
|
|
|
Well differentiated |
1.00 |
- |
- |
- |
|
Moderately differentiated |
2.63 |
1.74 |
3.95 |
0.049 |
|
Poorly differentiated |
4.10 |
3.01 |
6.82 |
< 0.001 |
|
Stage |
|
|
|
|
|
I |
1.0 |
- |
- |
- |
|
II |
1.95 |
1.22 |
3.11 |
0.024 |
|
III |
4.65 |
1.23 |
3.98 |
0.005 |
|
IV |
4.65 |
3.28 |
7.81 |
< 0.001 |
|
Morphology |
|
|
|
|
|
Ductal |
1.00 |
- |
- |
- |
|
Lobular |
0.41 |
0.34 |
1.47 |
0.757 |
|
Others |
1.43 |
0.45 |
2.67 |
0.414 |
|
Treatment |
|
|
|
|
|
Surgery |
1.00 |
- |
- |
- |
|
Chemotherapy |
3.91 |
2.14 |
7.12 |
< 0.001 |
|
Surgery and radiotherapy |
1.14 |
0.67 |
1.93 |
0.645 |
|
Surgery and chemotherapy |
1.20 |
0.61 |
2.38 |
0.595 |
CI: confidence interval.
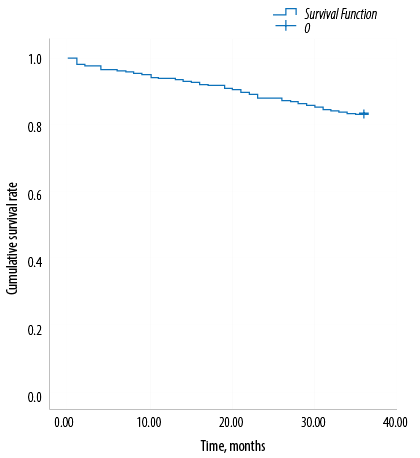
Figure 1: Overall observed survival of women with breast cancer.
Results
A total of 605 women with breast cancer were included in the study. The mean age of the patients at the time of diagnosis was 50.5±12.1 years (range 22–85 years). About two-thirds (63.1%) were aged < 55 years. The most frequent cancer type was IDC (89.8%). More than half (58.5%) of the patients were with grade 2 (moderately differentiated) breast cancers, while only 7.1% were with grade 1 (well differentiated). Similarly, only 45 (7.4%) patients presented with stage I breast cancer, whereas nearly half had advanced stages (stage III (30.2%) and stage IV (13.9%)) [Table 1].
The overall observed three-year survival rate of women with breast cancer in Basrah was 83.3% [Figure 1].
The cumulative three-year survival rates by the Kaplan-Meier method as a function of different prognostic factors (age, grade, stage, and morphology) are presented in Table 2. The highest three-year cumulative survival rate by age was among women aged 35–44 years, while the lower survival rates were among younger women (< 35 years) and those aged ≥ 55 years. Age was not a significantpredictor of survival (p = 0.068). According to the tumor grade, the survival rate was lowest for the poorly differentiated tumor compared to well differentiated tumors (71.6% vs. 93.0%, p < 0.001).
The stage of the tumor was adversely related to the survival rate and mean survival time. Stage I was associated with higher mean survival time (35.3 months) and survival rate (97.8%), while in women with stage IV breast cancer, the mean survival time was 27.3 months and the survival rate was 60.7%, (p < 0.001). Morphology was an insignificant predictor of survival (p = 0.528). However, patients with lobular carcinoma showed a better survival rate and mean survival time than those with other morphology types.
Cox proportional hazard regression analysis showed that patients with stage IV breast cancer had a worse prognosis than those with stage I (hazard ratio (HR) = 4.65; 95% confidence interval (CI): 3.28–7.81; p < 0.001). Similarly, patients with poorly differentiated tumors showed poorer prognosis compared with well differentiated tumors (HR = 4.10; 95% CI: 3.01–6.82; p < 0.001). The use of chemotherapy alone was a significant predictor of poor prognosis. All other types of treatment showed no significant effect on prognosis [Table 3].
Discussion
Many factors affect the interpretation of cancer survival including accuracy and completeness of cancer registration, the completeness of follow-up, tumor factors like stage and grade at diagnosis, and personal characteristics like age at diagnosis.24
This study showed that the overall three-year observed survival rate was 83.3%. This result is comparable to that reported in Saudi Arabia (85%)25 and Iran (82.4%).26 However, it is better than that reported in Jordan (70.2%)27 and Thailand (59.9%).28
Vast differences in breast cancer survival between countries have been reported, depending on the availability of screening and diagnostic facilities and advances in treatment modalities.29 One study investigated the three-year breast cancer survival in six high-income countries (Australia, Canada, Denmark, Norway, Sweden, and the UK) during 2000–2007 and showed that the age-standardized three-year net survival was 87–89% in the UK and Denmark, and 91–94% in the other four countries.30
The five-year survival rate ranges from more than 80% in developed countries (such as North America, Sweden, and Japan) to about 60% in middle-income countries and < 40% in low-income countries.31
Such variation in survival between developed and developing countries is attributed to the challenges that developing countries face in the application of the four key components to controlling cancer. These were stated by the World Health Organization and include cancer prevention, early detection, diagnosis, and treatment with palliation.32 In addition, other factors such as differences in population structure (life expectancy),33 low access to screening,34 lower socioeconomic status,35 low access to high-quality healthcare, poor treatment adherence,36 poor lifestyle modification after a cancer diagnosis,37 and variation in tumor biology contribute to this variation.38
A debate about the impact of young age at diagnosis on the survival of women with breast cancer has been long documented.39 Although younger women (< 35 years old) and older women (≥ 55 years old) showed a lower survival rate than those aged 35–44 years, this study showed that age was not associated with survival. This result agrees with that of many previous studies.26,40 On the contrary, many studies indicated younger age as a factor associated with poor survival.27,40 The age effect may be distorted by the fact that the outcome was death from all causes rather than breast cancer deaths. It is also likely that the follow-up time is too short to document such an age effect.
The results of this study showed that the three-year survival rate was significantly poorer in patients with stage IV breast cancer than in patients with
stage I. These results are in agreement with that reported in both developed and developing countries. In developed countries, despite the advances in biomedical researches and early detection and treatment, stage IV accounts for a large proportion of mortality.41
In developing countries including Iraq, women with breast cancer usually presented with advanced stage and consequently poor prognosis.42 In Jordan, the five-year survival for stage IV patients was 5.8% vs. 96% for those with stage I breast cancer.27 A study in Thailand showed that the five-year survival for early stage cancer was (60%; 95% CI: 0.51–0.67) higher than the late stage (27%; 95% CI: 0.19–0.34).28 In Pakistan, the five-year stage-specific survival was 100%, 88%, 58% for stage I, II, III respectively, and the median survival for stage IV patients was 18 months.43
This study showed that there was a significant association between the grade of breast cancer and survival, which has been reported previously.43 Patients with grade 3 (poorly differentiated tumors) had an increased mortality risk compared to those with grade 1 (well differentiated) (HR = 4.10; 95% CI: 3.01–6.82; p < 0.001).
In agreement with a study done in Jordan,27 tumor morphology showed no statistically significant effect on survival. Nevertheless, the lobular subtype of breast cancer was found to be less aggressive than other types of breast cancer in accordance with previous reports.44
In agreement with what was previously reported in Jordan,27 Brazil,45 and Iran24 patients who were surgically treated showed better survival than those treated with combined therapy of surgery, radiotherapy, and chemotherapy but without significant difference. Contrarily to that reported in Oman,42 chemotherapy alone was associated with a worse prognosis. Chemotherapy or medical treatment is mostly considered in metastatic breast cancer; therefore, it may reflect the severity of cancer, which is responsible for the high risk of death.46
Several limitations of this study should be addressed. First, incompleteness in death registries cannot be excluded entirely. However, death is a recognizable event, and the fact of death is usually ascertained in Islamic countries for legal, religious, and social issues (such as a proof for burial or inheritance claims).47 Some inaccuracies may occur about the cause of death, particularly in elderly people. In this study, the aim was not to calculate the cause-specific death rate, but to calculate mortality among women with breast cancer regardless of the cause. Therefore, the effect of such a limitation might be reduced. Being a registry-based study, many prognostic factors such as PR, ER, HER2/neu statuses, socioeconomic status, and educational level were completely missing. Furthermore, information on occupation was either incomplete or written incorrectly, so these variables were not analyzed. Therefore, their influence on survival will be difficult to gauge. The strength of the study is that it was the first to assess the survival rate of breast cancer in Basrah.
Conclusion
The three-year survival rate of women with breast cancer in Basrah was comparable to that in some developing countries. However, it was poorer than that in developed countries. Advanced stage and grade of the tumor were significantly associated with poor survival. Health care providers must be more aware of different treatment modalities used to treat cancer in addition to the development of tertiary care to improve the survival of patients with breast cancer. Educational programs for women about breast self-examination and implementation of screening programs are vital for early detection of breast cancer. Further studies are needed to ensure the inclusion of other variables such as lymph node status ER, PR, and HER2/neu expression.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015 Mar;136(5):E359-E386.
- 2. World Health Organization, International Agency for Research on Cancer. Latest world cancer statistics. PR 223, 2013 [cited 2019 January 5]. Available from: https://www.iarc.fr/en/media-centre/pr/2013/pdfs/pr223_E.pdf.
- 3. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010 Aug;19(8):1893-1907.
- 4. Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med 2014 Jun;11(2):101-115.
- 5. Shulman LN, Willett W, Sievers A, Knaul FM. Breast cancer in developing countries: opportunities for improved survival. J Oncol 2010;2010:595167.
- 6. Ministry of Health. Iraq. Annual report Iraqi cancer registry 2015, Iraqi Cancer Board, 2018 [cited 2019 January 10]. Available from: https://www.moh.gov.iq/upload/upfile/ar/833.pdf.
- 7. Hussain RA, Habib OS. Incidence of cancer in Basrah: results of a household survey. Asian Pac J Cancer Prev 2015;16(1):163-167.
- 8. S Habib O, A Hameed L, A Ajeel N, Al-Hawaz MH, Al-Faddagh ZA, N Nasr G, et al. Epidemiology of breast cancer among females in Basrah. Asian Pac J Cancer Prev 2016;17(S3):191-195.
- 9. Habib SH, Al-Ali JK, Al-Wiswasi MK, Ajeel NA. The burden of cancer in Basrah: The state of art- first report 2006 [cited 2019 January 20]. Available from: https://www.muhadharaty.com/files/lectures/009/file8736.pdf.
- 10. Karimi A, Delpisheh A, Sayehmiri K, Saboori H, Rahimi E. Predictive factors of survival time of breast cancer in kurdistan province of Iran between 2006-2014: a cox regression approach. Asian Pac J Cancer Prev 2014;15(19):8483-8488.
- 11. Kantelhardt EJ, Zerche P, Mathewos A, Trocchi P, Addissie A, Aynalem A, et al. Breast cancer survival in Ethiopia: a cohort study of 1,070 women. 2014;135:702–709.
- 12. Shandiz FH, Shabahang H, Afzaljavan F, Sharifi N, Tavasoli A, Afzalaghaee M, et al. Ki67 Frequency in breast cancers without axillary lymph node involvement and its relation with disease-free survival. Asian Pac J Cancer Prev 2016;17(3):1347-1350.
- 13. Britten A, Rossier C, Taright N, Ezra P, Bourgier C. Genomic classifications and radiotherapy for breast cancer. Eur J Pharmacol 2013 Oct;717(1-3):67-70.
- 14. Chen H-L, Ding A, Wang F-W. Prognostic effect analysis of molecular subtype on young breast cancer patients. Chin J Cancer Res 2015 Aug;27(4):428-436.
- 15. Rossi L, Laas E, Mallon P, Vincent-Salomon A, Guinebretiere JM, Lerebours F, et al. Prognostic impact of discrepant Ki67 and mitotic index on hormone receptor-positive, HER2-negative breast carcinoma. Br J Cancer 2015 Sep;113(7):996-1002.
- 16. Cockburn JG, Hallett RM, Gillgrass AE, Dias KN, Whelan T, Levine MN, et al. The effects of lymph node status on predicting outcome in ER+ /HER2- tamoxifen treated breast cancer patients using gene signatures. BMC Cancer 2016 Jul;16:555.
- 17. Hislop GT, Bajdik CD, Regier MD, Barroetavena MC. Ethnic differences in survival for female cancers of the breast, cervix and colorectum in British Columbia, Canada. Asian Pac J Cancer Prev 2007 Apr-Jun;8(2):209-214.
- 18. World Health Organization. International statistical classification of diseases and related health problems. ICD-10. Geneva; 2016 [cited 2019 February 10]. Available from: http://apps.who.int/classifications/icd10/browse/2016/en.
- 19. Ellis IO, Schnitt SJ, Sastre-Garau X, Bussolati G, Tavassoli FA, Eusebi V, et al. Invasive breast carcinoma. In: Tavassoli FA, Devilee P, editors. World Health Organization Classification of tumors. Pathology and genetics. Tumors of the breast and female genital organs. Lyon: International Agency for Research on Cancer 2003. p. 18-19 [cited 2019 February 8]. Available from: https://www.iarc.fr/en/publications/pdfs-online/pa t-gen/bb4/BB4.pdf.
- 20. National Comprehensive Cancer Network. Clinical practice guidelines in oncology. Breast Cancer 2016;[cited 2019 February 18]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 21. Going J. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Department of Pathology, Western Infirmary, Glasgow. NHS Cancer Screening Programmes 2005[cited 2019 March 1]. Available from: https://ar.scribd.com/document/266601308/Breast-Cancer-Grading-pdf.
- 22. Porta M, Greenland S, Hernán M, Silva I, Last J, Burón A. A Dictionary of Epidemiology, 6th edition, Oxford University Press, New York, 2014 [cited 2019 March 1]. Available from:: http://irea.ir/files/site1/pages/dictionary.pdf.
- 23. Davoudi Monfared E, Mohseny M, Amanpour F, Mosavi Jarrahi A, Moradi Joo M, Heidarnia MA. Relationship of social determinants of health with the three-year survival rate of breast cancer. Asian Pac J Cancer Prev 2017 Apr;18(4):1121-1126.
- 24. Fouladi N, Amani F, Harghi AS, Nayebyazdi N. Five year survival of women with breast cancer in Ardabil, north-west of Iran. Asian Pac J Cancer Prev 2011;12(7):1799-1801.
- 25. Abou Zaid LZ, Nuzhat A, Rafiqe M. Factors affecting survival of women with breast cancer in King Fahad Medical City, Saudi Arabia. Int J Community Med Public Health 2017;4:910-915.
- 26. Abedi G, Janbabai G, Moosazadeh M, Farshidi F, Amiri M, Khosravi A. Survival rate of breast cancer in Iran: a meta-analysis. Asian Pac J Cancer Prev 2016 Oct;17(10):4615-4621.
- 27. Arkoob K, Al-Nsour M, Al-Nemry O, Al-Hajawi B. Epidemiology of breast cancer in women in Jordan: patient characteristics and survival analysis. East Mediterr Health J 2010 Oct;16(10):1032-1038.
- 28. Poum A, Kamsa-ard S, Promthet S. Survival rates of breast cancer: a hospital-based study from northeast of Thailand. Asian Pac J Cancer Prev 2012;13(3):791-794.
- 29. Møller H, Sandin F, Bray F, Klint A, Linklater KM, Purushotham A, et al. Breast cancer survival in England, Norway and Sweden: a population-based comparison. Int J Cancer 2010 Dec;127(11):2630-2638.
- 30. Walters S, Maringe C, Butler J, Rachet B, Barrett-Lee P, Bergh J, et al; ICBP Module 1 Working Group. Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000-2007: a population-based study. Br J Cancer 2013 Mar;108(5):1195-1208.
- 31. Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, et al; CONCORD Working Group. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 2008 Aug;9(8):730-756.
- 32. Sharma V, Kerr SH, Kawar Z, Kerr DJ. Challenges of cancer control in developing countries: current status and future perspective. Future Oncol 2011 Oct;7(10):1213-1222.
- 33. Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat 2011 Jun;127(3):729-738.
- 34. Schopper D, de Wolf C. How effective are breast cancer screening programmes by mammography? Review of the current evidence. Eur J Cancer 2009 Jul;45(11):1916-1923.
- 35. Kaffashian F, Godward S, Davies T, Solomon L, McCann J, Duffy SW. Socioeconomic effects on breast cancer survival: proportion attributable to stage and morphology. Br J Cancer 2003 Nov;89(9):1693-1696.
- 36. Yip CH, Taib NA, Mohamed I. Epidemiology of breast cancer in Malaysia. Asian Pac J Cancer Prev 2006 Jul-Sep;7(3):369-374.
- 37. Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA 2005 May;293(20):2479-2486.
- 38. Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 2007;9(1):R6.
- 39. Rezaianzadeh A, Peacock J, Reidpath D, Talei A, Hosseini SV, Mehrabani D. Survival analysis of 1148 women diagnosed with breast cancer in Southern Iran. BMC Cancer 2009 Jun;9:168.
- 40. Seedhom AE, Kamal NN. Factors affecting survival of women diagnosed with breast cancer in El-Minia Governorate, Egypt. Int J Prev Med 2011 Jul;2(3):131-138.
- 41. Lim B, Hortobagyi GN. Current challenges of metastatic breast cancer. Cancer Metastasis Rev 2016 Dec;35(4):495-514.
- 42. Kumar S, Burney IA, Al-Ajmi A, Al-Moundhri MS. Changing trends of breast cancer survival in sultanate of oman. J Oncol 2011;2011:316243.
- 43. Kumar S, Shaikh AJ, Rashid YA, Masood N, Mohammed A, Malik UZ, et al. Presenting features, treatment patterns and outcomes of patients with breast cancer in Pakistan: Experience at a university hospital. Indian J Cancer 2016;53:230-234.
- 44. Yang LP, Sun HF, Zhao Y, Chen MT, Zhang N, Jin W. Clinicopathological characteristics and survival outcomes in pleomorphic lobular breast carcinoma of the breast: a SEER population-based study. Cancer Med 2017 Dec;6(12):2867-2875.
- 45. Fujimoto RH, Koifman RJ, Silva IF. Survival rates of breast cancer and predictive factors: a hospital-based study from western Amazon area in Brazil. Cien Saude Colet 2019 Jan;24(1):261-273.
- 46. Pierga JY, Mouret E, Diéras V, Laurence V, Beuzeboc P, Dorval T, et al. Prognostic value of persistent node involvement after neoadjuvant chemotherapy in patients with operable breast cancer. Br J Cancer 2000 Dec;83(11):1480-1487.
- 47. Essa SS, Habib OS, Al-Diab JM, Al-Imara KA, Ajeel NA. Cancer mortality in Basrah. Med J Basrah Univ 2007;25:55-60.