Spermatogenesis is a highly synchronized, regular, and lengthy process in which sperm are formed from germ cells which gradually differentiate into mature and haploid cells called spermatozoa.1,2 During this process, sperm become sensitive to several extrinsic and intrinsic stressors, including a variety of diseases, hormonal disorders, genetic factors, and mutagen processes.3,4 According to previous statistical findings, 35% of infertility cases are related to men while 40% are related to women. The most common reason for male infertility is their inability to produce enough healthy and active sperm cells.4,5 Exposure to external factors before and after birth and during the newborn stages could jeopardize their infants and their productive ability.6 These factors could have an impression on spermatogenesis, spermiogenesis, sperm motility, sperm chromatin, and changes in DNA integrity as well as hormonal regulation in fertility.7,8 During the past decades, there has been a marked decline in sperm quality and fertility. Therefore, it is obvious that sperm quality is affected by changes, which are rooted in toxic agents that exist in the human environment.8 A wide range of wavelengths are today emitted via radar, communication instruments, mobile phone base stations, high voltage lines, TV and radio transmitters, the electrical substations, and electrical devices at home and work such as a phones, computers (laptops), or flashlights.9 Furthermore, the electromagnetic spectrum ranges in very broad frequency, which include very low frequencies, radiofrequency (RF) and infrared, ultraviolet radiation, X-rays, and gamma rays.8,10,11 Magnetic resonance imaging (MRI) as a new imaging method, due to lack of ionizing radiation, high spatial resolution, and low scanning time is one of the best diagnostic systems to survey and monitor the status of various diseases and provide valuable information about anatomical structure, as well as the physiological and metabolic status of organs.12 In this method, the three main fields include the main magnetic field (B0), the RF and various magnetic field gradients with interval time to produce images inside the body.13 Triple-frequency fields depend on the type of system, for example, the 0.5 Tesla system uses 21 MHz RF waves and the 1.5 Tesla system uses 64 MHz.12,13 MRI hazards can occur as a negative feedback of any of these fields. Although the biological effects of these fields have been studied separately in many literature reports, but information about the potential risks of combining all three fields on tissue is low.13,14 Several studies have reported the effects of each of these fields on biological tissues. A steady field can cause a delay in growth and withdrawal of calcium from the neural membranes, and prevents normal metabolism.15,16 RF fields can also increase the permeability of the blood-brain barrier, produce free radicals, ionic transport, chromosomal aberrations, and changes in some membrane proteins within the cell.15–17Among the biological effects of the time-varying gradient field (TVMF), stimulation of the cardiac conduction system and the peripheral nerve have been mentioned.16 According to the reports of experiments that were conducted on animal models, waves of MRI 0.35 Tesla to the mice in the middle of pregnancy could reduce the crown-rump length (CRL) of the embryo.18 Exposure to B0 and TVMF has no negative effect on mortality, the amount of hatching, and survival chick embryos in one study.19 However, exposure of 1.5 Tesla can significantly increase the abnormalities and mortality of embryos at the sixth day after exposure.20 Other researchers also reported that the exposure of pregnant mice by utilizing a constant magnetic field of 4.7 Tesla for eight hours on days nine and 12 of pregnancy reduces fetal weight, the number of sperm, and CRL, while the rate of stillbirth increased.13 Another study reported that MRI exposure (1.5 Tesla) resulted in eye abnormalities during development between 15 to 27% higher than the control group.21 According to previous studies, each of the fields used in MRI caused some destructive bio-effects on organisms. The amount of negative feedback of these effects depend on field strength, exposure time, frequency, and the tissues tested.15,22 According to the law of Bergonié and Tribondeau young, fundamental and immature cells are more sensitive to radiation, particularly during division.23 Since high-volume imaging assays have increased around the world, unnecessary MRI requests for patients have been enhanced. Also, numerous people such as equipment engineers, medical imaging researchers, technical operator during injection of contrast media, the surgeons involved in carrying out a test are irradiated in all three MRI fields. Thus, the main purpose of this study was to investigate the effects of MRI on testicular histology and the morphometry of seminiferous tubules in mice.
Figure 1: (a) Placement and arrangement slice of mice in the MRI machine. (b) Image after scanning signals which indicate exposure of mice to form an image.
Methods
We used 40 adult Naval Medical Research Institute male mice aged six to eight weeks weighing 30.0±1.0 g, pathogen free. The animals were kept under standard conditions of light, temperature (25.0±2.0°C) and humidity (45–55%) for two weeks (12 dark/light cycle), and were allowed to have free access to food and water ad libitum. All procedures were performed according to the guidelines of the Ethical Committee of Kurdistan University of Medical Sciences, Sanandaj, Iran. The mice were randomly divided into two groups, 20 were exposed and the other 20 mice were sham exposed and kept in the off-set MRI scanner (mice did not receive any exposure and just the system was on stand-by and at the same time remained on the MRI bore). Mice were kept in a special holder individually and fixed, then each mouse was scanned separately under modified human imaging parameters. Mice were exposed to a MRI system over three weeks, once per week for 36 minutes [Figure 1]. After one hour of exposure, 10 of the sham mice were sacrificed (group I) as well as 10 exposed mice (group II). Thirty-five days later, 10 more mice from both the sham (group III) and exposed group were killed (group IV). The exposure time of 36 minutes was chosen as that is the duration time to overcome any problems the patient may have in the magnet room (i.e, claustrophobia, tachycardia), and for the operator to install the coils. In dynamic examination and contrast administration tests this time is higher than 36 minutes [Table 1].24 Furthermore, the MRI technician works 30 hours in the MRI ward weekly, 56% of this time the operator is exposed to both static magnetic field and TVMF.25 Thirty-five to forty minutes of exposure to MRI fields to the heart caused DNA aberrations in lymphocytes. Since the whole body was exposed to time static magnetic field, the pelvis and gonads were also exposed for 35–40 minutes.26 Exposure to RF waves from 1.5 Tesla MRI scanner for 36 minutes to female mice on day seven of gestation reduced the craniofacial perimeter and CRL in comparison to the sham exposed animals.27 We used the MRI scanner (Siemens Co., Magnetom ESSENZA, Germany) with field strength of 1.5 Tesla and RF range of 64 MHz to emit waves. During scanning, the gradient intensity was 23 mT/m and the specific absorption rate (SAR) was 0.15 W/kg. The SAR formula calculates absorbed energy in the exposed body tissue and is given by: SAR = P (1/M) × (τ/TR) = 190 w (1/1.2 kg) × (4 ms/4000 ms) = 0.15 W/kg. In which P is pulse power (in watts), M is the mass of the exposed material (in kg), τ is the pulse working factor (in milliseconds- ms), and TR is the duration time of each pulse (in ms). According to the Food and Drug Administration instructions, in this condition the additional temperature will not be transferred to the animal samples, because temperature rise for whole body exposure occurs in SAR range of 0.8 W/kg or higher.28 Finally, the testes were evaluated for both histological (light microscopy) and morphological changes of the seminiferous tubules.
The animals were weighed using a digital scale (Sartorius; model-BL210S) before being sacrificed. Thereafter, the peritoneal cavity was opened and their testes were weighed. Thus, at one hour and day 35 after exposure, 10 mice in each group were sacrificed by cervical dislocation and their testes were removed from the abdominal cavity. The samples were fixed in 10% formaldehyde buffer for 72 hours at room temperature followed by ethanol before finally being embedded in longitudinal axis into paraffin wax. The samples were then cut into 5 µM thick sections using a rotary microtome (Leitz, Germany). Five slides were prepared from each testis and were stained with hematoxylin and eosin (H&E staining; Sigma, USA) for light microscopy. To evaluate the maturity and quality of the seminiferous tubules, Johnson’s method was used.29 The Johnson scoring scale evaluates the quality and maturity of the tubule in each cross-section of the sample and scores them from 1 to 10 [Table 2].
For the quantitative evaluation of seminiferous tubules, a graded linear eyepiece lens was used. Twenty seminiferous tubules of each animal were randomly selected from a round cross-section. In each animal, 20 cells were studied. Morphologically oriented, spermatogonia, primary spermatocytes, round spermatids, and spermatozoa in 20 seminiferous tubules were counted per animal. Tubules which were elliptical or had diagonal cutting were excluded. The basal membrane of the tubules was calculated from one hand to the other side of the basement. At first, two perpendicular diameters were (minor and major axes) calculated and followed by the means of diameter in each tubule.30 Similarly, the mean of diameters of the lumen of the seminiferous tubule and epithelium thickness was measured using the Image J software (Version-1.34, National Institute of Health, and Bethesda, MD, USA).
For quantitative assessment, the ANOVA and Tukey tests were used for follow-up (statistical analysis) and the results are presented as number and percentage. All statistical tests were performed using SPSS Statistics (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp.). A p-value < 0.050 was considered statistically significant.
Table 1: Modified parameters and pulse sequences for male pelvic imaging in mice by MRI scanner.
|
Scout |
GE |
Sagittal, transverse, and coronal |
6 |
15 |
30 |
45 × 45 |
256 × 256 |
10 |
3 |
1 |
|
GE with fat suppression |
GE |
Transverse |
4.1 |
148 |
80 |
35 × 26 |
256 × 256 |
8 |
20 |
1 |
|
GE with fat suppression |
GE |
Sagittal |
4.1 |
148 |
80 |
35 × 26 |
256 × 256 |
7 |
20 |
1 |
|
High resolution T2-weighted Turbo SE |
Turbo SE |
Sagittal |
132 |
4902 |
180 |
35 × 26 |
512 × 512 |
5 |
19 |
2 |
|
High resolution T2-weighted Turbo SE |
Turbo SE |
Transverse |
132 |
4902 |
180 |
35 × 26 |
512 × 512 |
5 |
19 |
2 |
|
Half acquisition Turbo SE |
Half acquisition Turbo SE |
Transverse |
90 |
4.4 |
150 |
35 × 26 |
256 × 256 |
8 |
20 |
1 |
TE: echo time; TR: repeat time; FA: flip angle; FOV: field of view; GE: gradient echo; SE: spin echo; NEX (NSA): number of excitations
(number of signal averages).
Table 2: Qualitative assessment of fertility and the spermatogenesis process according to Johnson’s scoring scale.
|
1 |
Germ and Sertoli cells cannot be seen. Tubules are atrophic. |
|
2 |
There are no germ cells, only Sertoli cells can be seen. |
|
3 |
There are no primary spermatocytes. Just spermatogonia can been seen. |
|
4 |
Very few primary spermatocytes can be seen. |
|
5 |
There is no sperm and round spermatid. A large number of primary spermatocytes can be seen. |
|
6 |
A few round spermatids can be seen. |
|
7 |
There is no sperm; however, a large number of round spermatids are visible. |
|
8 |
Sperm count is very low. |
|
9 |
There is a large number of sperm but round sperm cannot be seen and the lumen has no regular contour. |

Figure 2: (a) Group I: The basal membrane of the seminiferous tubule is seen and sperm cell lines are present. Leydig cells have a normal view in the interstitial tissue (hematoxylin and eosin (H&E) staining, magnification = 400 ×). (b) Group II: The diameter of the seminiferous tubules decreased slightly and induced the partial tissue edema (H&E staining, magnification = 400 ×).
Table 3: Results obtained from Johnson scoring and testicular weight after last irradiation in the all groups. Data represented as means and standard deviation.
|
I |
9.1 ± 0.2 |
0.11 ± 0.01 |
|
II |
8.7 ± 0.4 |
0.10 ± 0.01 |
|
III |
9.3 ± 0.8 |
0.1 ± 0.1 |
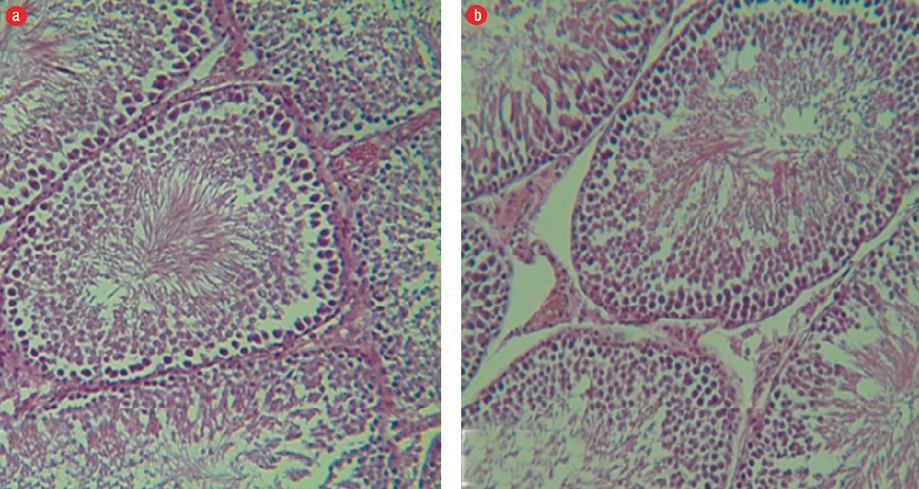
Figure 3: (a) Group III: Seminiferous tubules and germinal epithelium with normal, regular and well-organized size. The germinal epithelium in the seminiferous tubules is normal and desired, and all lines of sperm and Sertoli cells are visible in the epithelium of the seminiferous tubule (hematoxylin and eosin (H&E) staining, magnification = 400 ×). (b) Group IV: Seminiferous tubules, sperm cell lines, and basal membrane can be clearly seen (H&E staining, magnification = 400 ×).
Table 4: The number of germ cells in the seminiferous tubules after irradiation by MRI in different groups. Data represented as means and standard deviation.
|
I |
3.0 ± 0.6 |
57.0 ± 3.7 |
64.5 ± 8.0 |
80.2 ± 6.7 |
|
II |
2.9 ± 1.1 |
51.3 ± 5.1 |
60.0 ± 6.2 |
78.1 ± 8.6 |
|
III |
3.3 ± 1.0 |
56.5 ± 2.7 |
64.4 ± 5.1 |
80.0 ± 5.2 |
Table 5: Radiation effects from MRI on histomorphological parameters in seminiferous tubules. Data represented as means and standard deviation.
|
I |
153.2 ± 0.7 |
79.3 ± 1.0 |
66.8 ± 0.4 |
|
II |
146.0 ± 1.5 |
78.7 ± 4.1 |
64.0 ± 1.3 |
|
III |
150.9 ± 1.2 |
80.05 ± 0.04 |
65.2 ± 2.8 |
Results
We observed regular and integrated seminiferous tubules, and the presence of all sperm cell lines in the testes of group I mice. The basal membrane in the seminiferous tubules was seen and spermatogonia cell lines were found on them. In the interstitial tissue of Leydig cells, cytoplasm acidophilic forms were completely observed. Spermatogenesis was visible and the arrangement of cells had a specific complete form and were well-organized [Figure 2a]. In group II, we observed a slight edema and inflammation of the tissues. There was a decrease in Leydig cells of the amount of interstitial tissues, and their cytoplasm was basophilic indicating a relative decrease in the thickness of the epithelium, and sparse scattering was associated with increasing interstitial space [Figure 2b]. In groups III and IV, we observed intact tubules and interstitial tissue with normal appearance, and seminiferous tubules were observed regularly and completely. All sperm cell lines were seen, and the basal membrane in the seminiferous tubules was normal and spermatogonia stem cells were on it. The Leydig cells in the interstitial tissue were observed to have a slightly acidophilic cytoplasm. Spermatogenesis was visible and the proliferation of spermatogonia increased during rest [Figures 3 a and b].
The weight of mice testes at one hour after the last exposure in group II partially decreased compared with the control group (group I), but this was not statistically significant [Table 3]. Thirty-five days after the last exposure, there was no significant difference between the exposed and the control groups (p = 0.730). Spermatogenesis in the control and experimental groups was evaluated based on Johnson’s scoring. The results indicated that in group I, the spermatogenesis average total score (score 10) was 9.1. The score in groups II, III and IV were 8.7, 9.3, and 9.0, respectively [Table 3].
The results revealed that exposure causes reduction in the number of spermatogonia in group II compared with group I, but there was no significant difference (p = 0.086). There was no cell loss or destruction in primary spermatocytes and round spermatid cell lines in group II compared to group I (p = 0.610 and p = 0.470, respectively). In group IV, the primary spermatocytes were partly increased, which indicate slight totally reversible changes induced by emitted MRI waves. The effects of these waves after a period of spermatogenesis (35 days) on round spermatid cells was not found to be significant (p = 0.260). Moreover, the number of spermatozoa negligibly decreased in group II compared to group I, but it was not significant (p = 0.370) [Table 4]. The results also showed that radiowaves from MRI cause partial changes in the histomorphological parameters of seminiferous tubules, such that the diameter of the tubules in group II mice decreased in comparison with group I mice, but was not significant (p = 0.730). In contrast, there was no substantial change in the morphology of the lumen diameter after exposure of group II mice (p = 0.920). The thickness of the epithelium as well as other important morphometric parameters were investigated, and in group II there was no observed adverse effect from exposure to radiowaves (p = 0.860) [Table 5].
Discussion
With the rapid development of wireless communication devices, there is widespread concern about the possible adverse consequences of exposure to electromagnetic fields (EMFs) on health. For instance, empirical studies have reported that the RF EMF from cell towers and wireless devices can disrupt neurotransmitter balance, blood-brain barrier cellular metabolism, regulation of calcium efflux systems activity, and gene and protein expression in different kinds of cells even at a low frequency.31 The biological effects of EMF are mediated by generation of reactive oxygen species (ROS) and free radicals. An elevation of toxic free radicals, leading to resultant stress and damage to cellular systems, as oxidative stress.9 Different internal or external factors, including gamma or ultraviolet radiation, can be induced oxidative stress. Also it has been demonstrated that EMFs at extremely low frequencies are capable of increasing free radical production including hydroxyl free radicals, which can cause the formation of strand breaks in cellular DNA.32 In addition, the effects of exposure to RF-EMFs from cellphones on the reproductive function have been the subject of a large number of studies in recent years. Some results confirm that heavy long-term use of cellphones could have adverse effects on fertility and the reproductive system.11,33–35
Our study showed that the emission of electromagnetic waves with MRI 1.5 Tesla scanner for 36 minutes once a week for three consecutive weeks had partial effects on the qualitative and quantitative parameters of testicular tissue. It affected testicular weight, and also caused a minor delay in the spermatogenesis process. These changes were time-dependent and reversible, especially after restarting the spermatogenesis period. A previous study reported that mice exposed to 4.7 Tesla recorded a significant increase in the number of dead embryos, and the daily production of sperm was noticeably reduced.36 Also, a 1996 study reported that the exposure of male mice to a static magnetic field (1.5 Tesla) for 30 minutes led to a reduction in the number of sperm and significant abnormalities in the morphology of the sperm’s head. These researchers also showed that the survival of irradiated embryos in the two-cell stage was 0.56% less compared to the control group and is consistent with our findings.37
In addition to the existing effects of MRI fields, many other studies regarding EMF have been conducted on fertility including the impact of weak static magnetic fields, varying-time of magnetic field exposure, and the RF. These EMFs are present in equipment such as high voltage cables38 and cellphones.39 Studies have revealed that waves produce free radicals, which in turn cause phosphorylation and activation of some messenger proteins such as histone kinase and creatine kinase. As a result of these changes, the rate of ROS production significantly increased and activates the caspase-3 pathway in sperm cells (due to apoptotic death during spermatogenesis or a period of sperm maturation occur) and can affect the fertility capacity and output of the sperm cells.40 In 2011, a study looked at the effects of a magnetic field of 1 Gauss (50 Hz) on the fertility of male rats for 21 days. One group was examined immediately after wave emission, and the other group was examined 48 days after the last exposure (the duration of spermatogenesis in rats), and it was seen that testicular weight and the number of sperm in the exposed group in comparison with the control group had significantly decreased, but these changes returned to normal after 48 days. The researchers showed that the magnetic field is deleterious for testicular function and induced oxidative stress on spermatogenesis.41 The results are consistent with our study with respect to reversible changes after a period of spermatogenesis (35 days).
Another potential factor that caused stress in our study during scanning was the MRI noises that were emitted from three gradients. The simultaneous emission of noise and RF waves can be an important factor for the decrease in sperm parameters such as the number, viability, and morphology, as well as antioxidant capacity and the reproductive capacity in Wistar rats.42 During a typical scan, the range of noises emitted from the MRI 1.5 Tesla scanner usually varies between 101.8 to 111.7 dB.43 Emitting a noise of 100 dB to an albino rat for one hour per day in 60 days resulted in a significant decrease in the serum levels of testosterone, destruction in the structure of seminiferous tubules, arrest at the germinal layers and disruption in the basal membrane of the testicular tissue.44 Therefore, in our study a decrease was recorded in testis weight, lumen and tubule diameter, and the thickness of the epithelium. This could have resulted from the negative effect of noise gradients. It can be concluded that minor changes in the testis tissue (as seen in groups I and II) can also be caused by environmental stress and intermittent MRI noise in the stand-by mode. Male mice exposed for three hours per day, six days per week for eight weeks to 50Hz EMFs with a 0.5 Tesla intensity were found to have a reduction in the thickness of the walls of the epididymis and vas deferens, and a decreased height of epithelial cells and testicular weight when compared with the control group similar to our findings.45 In this regard, specific and detailed instructions should be provided since a wide range of medical personnel including medical engineering staff, medical imaging researchers, crews, and technical operators during injection of contrast material in the dynamic tests are exposed to all three MRI fields.46 As a result of the high-volume imaging exams in the world (20 million per year more than fifty-thousand tests daily MRI) as well as the unnecessarily increased MRI orders for patients.47
Conclusion
EMFs from different wireless communicating devices and medical equipment potentially may negatively impact fertility by causing temporary or permanent fertility problems. However, the exact mechanisms of EMFs effects on cell biology and structural changes in tissue remain unclear. Our results indicate that exposure of mice to EMF at 1.5 Tesla for 36 minutes (once a week for three consecutive weeks) induces slight changes in the testicular tissues. These changes also have negative effects on spermatogenesis and leads to decrease sperm count, sperm motility, sperm survival and increased abnormal sperm morphology in male mice. Moreover, all these effects were reversible after a new period of spermatogenesis. However, to verify these effects and clarify the mechanisms involved, further studies are necessary using a longer exposure time and higher dose. Finally, various antioxidant prescription is recommended as an approach to protection of people against exposure to EMF.
Disclosure
The authors declared no conflicts of interest. This work was supported by Kurdistan University of Medical Sciences (KUMS), Sanandaj, Iran (Ethical Code # IR.MUK.REC.1396.2). The authors would like to thank MUK for providing
financial support.
references
- 1. Fatehi D, Mohammadi M, Shekarchi B, Shabani A, Seify M, Rostamzadeh A. Radioprotective effects of Silymarin on the sperm parameters of NMRI mice irradiated with γ-rays. J Photochem Photobiol B 2018 Jan;178:489-495.
- 2. Rostamzadeh A, Ghadimi T, Allahveisi A, Mohammadi M, Rezaei S, Rezaie MJ. The expression of Bax protein in the early stages of spinal cord injury in the sperm cells of rats. Polish Annals of Medicine/Rocznik Medyczny 2018;25(2).
- 3. Petrelli G, Mantovani A. Environmental risk factors and male fertility and reproduction. Contraception 2002 Apr;65(4):297-300.
- 4. Khaki A, Fatemeh F, Nouri M, Khaki AA, Ozanci CC, Ghafari-Novin M, et al. The effects of ginger on spermatogenesis and sperm parameters of rat. International Journal of Reproductive BioMedicine 2009;7(1):7-12.
- 5. Olea N, Fernandez MF. Chemicals in the environment and human male fertility. Occup Environ Med 2007 Jul;64(7):430-431.
- 6. Wong EW, Cheng CY. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci 2011 May;32(5):290-299.
- 7. Kumar S, Kumari A, Murarka S. Lifestyle factors in deteriorating male reproductive health. Indian J Exp Biol 2009 Aug;47(8):615-624.
- 8. Miyamoto T, Tsujimura A, Miyagawa Y, Koh E, Namiki M, Sengoku K. Male infertility and its causes in human. Adv Urol 2012;2012:384520.
- 9. Kıvrak EG, Yurt KK, Kaplan AA, Alkan I, Altun G. Effects of electromagnetic fields exposure on the antioxidant defense system. J Microsc Ultrastruct 2017 Oct-Dec;5(4):167-176.
- 10. Khadrawy YA, Ahmed NA, Ezz HS, Radwan NM. Effect of electromagnetic radiation from mobile phone on the levels of cortical amino acid neurotransmitters in adult and young rats. Rom J Biophys 2009;19(4):295-305.
- 11. Fatehi D, Anjomshoa M, Mohammadi M, Seify M, Rostamzadeh A. Biological effects of cell-phone radiofrequency waves exposure on fertilization in mice; an in vivo and in vitro study. Middle East Fertil Soc J 2018;23(2):148-153 .
- 12. Novotny Jr EJ. Metabolic brain imaging by magnetic resonance. Future Neurology 2006;1(5):659-663.
- 13. De Wilde JP, Rivers AW, Price DL. A review of the current use of magnetic resonance imaging in pregnancy and safety implications for the fetus. Prog Biophys Mol Biol 2005 Feb-Apr;87(2-3):335-353.
- 14. Schenck JF. Physical interactions of static magnetic fields with living tissues. Prog Biophys Mol Biol 2005 Feb-Apr;87(2-3):185-204.
- 15. Hartwig V, Giovannetti G, Vanello N, Lombardi M, Landini L, Simi S. Biological effects and safety in magnetic resonance imaging: a review. Int J Environ Res Public Health 2009 Jun;6(6):1778-1798.
- 16. Schaefer DJ, Bourland JD, Nyenhuis JA. Review of patient safety in time-varying gradient fields. J Magn Reson Imaging 2000 Jul;12(1):20-29.
- 17. Formica D, Silvestri S. Biological effects of exposure to magnetic resonance imaging: an overview. Biomed Eng Online 2004 Apr;3(1):11.
- 18. Heinrichs WL, Fong P, Flannery M, Heinrichs SC, Crooks LE, Spindle A, et al. Midgestational exposure of pregnant BALB/c mice to magnetic resonance imaging conditions. Magn Reson Imaging 1988 May-Jun;6(3):305-313.
- 19. Behr KP, Tiffe HW, Hinz KH, Lüders H, Friederichs M, Ryll M, et al. [The effect of magnetic resonance treatment on chicken embryos]. Dtsch Tierarztl Wochenschr 1991 Apr;98(4):149-152.
- 20. Yip YP, Capriotti C, Talagala SL, Yip JW. Effects of MR exposure at 1.5 T on early embryonic development of the chick. J Magn Reson Imaging 1994 Sep-Oct;4(5):742-748.
- 21. Tyndall DA, Sulik KK. Effects of magnetic resonance imaging on eye development in the C57BL/6J mouse. Teratology 1991 Mar;43(3):263-275.
- 22. Kangarlu A, Robitaille PM. Biological effects and health implications in magnetic resonance imaging. Concepts in Magnetic Resonance Part A 2000;12(5):321-359.
- 23. Beltrán-Pardo E, Jönsson KI, Wojcik A, Haghdoost S, Harms-Ringdahl M, Bermúdez-Cruz RM, et al. Effects of ionizing radiation on embryos of the tardigrade Milnesium cf. tardigradum at different stages of development. PLoS One 2013 Sep;8(9):e72098.
- 24. Zand KR, Reinhold C, Haider MA, Nakai A, Rohoman L, Maheshwari S. Artifacts and pitfalls in MR imaging of the pelvis. J Magn Reson Imaging 2007 Sep;26(3):480-497.
- 25. de Vocht F, Muller F, Engels H, Kromhout H. Personal exposure to static and time-varying magnetic fields during MRI system test procedures. J Magn Reson Imaging 2009 Nov;30(5):1223-1228.
- 26. Lancellotti P, Nchimi A, Delierneux C, Hego A, Gosset C, Gothot A, et al. Biological effects of cardiac magnetic resonance on human blood cells. Circ Cardiovasc Imaging 2015 Sep;8(9):e003697.
- 27. Tyndall DA. MRI effects on craniofacial size and crown-rump length in C57BL/6J mice in 1.5T fields. Oral Surg Oral Med Oral Pathol 1993 Nov;76(5):655-660.
- 28. Magin RL, Lee JK, Klintsova A, Carnes KI, Dunn F. Biological effects of long-duration, high-field (4 T) MRI on growth and development in the mouse. J Magn Reson Imaging 2000 Jul;12(1):140-149.
- 29. Johnsen SG. Testicular biopsy score count–a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1970;1(1):2-25.
- 30. Orazizadeh M, Khorsandi LS, Hashemitabar M. Toxic effects of dexamethasone on mouse testicular germ cells. Andrologia 2010 Aug;42(4):247-253.
- 31. Sivani S, Sudarsanam D. Impacts of radio-frequency electromagnetic field (RF-EMF) from cell phone towers and wireless devices on biosystem and ecosystem-a review. Biol Med (Aligarh) 2012;4(4):202-216.
- 32. Saliev T, Begimbetova D, Masoud AR, Matkarimov B. Biological effects of non-ionizing electromagnetic fields: Two sides of a coin. Prog Biophys Mol Biol 2019;141:25-36.
- 33. Al-Quzwini OF, Al-Taee HA, Al-Shaikh SF. Male fertility and its association with occupational and mobile phone towers hazards: An analytic study. Middle East Fertil Soc J 2016;21(4):236-240.
- 34. Al-Bayyari N. The effect of cell phone usage on semen quality and fertility among Jordanian males. Middle East Fertil Soc J 2017;22(3):178-182.
- 35. Adams JA, Galloway TS, Mondal D, Esteves SC, Mathews F. Effect of mobile telephones on sperm quality: a systematic review and meta-analysis. Environ Int 2014 Sep;70:106-112.
- 36. Carnes KI, Magin RL. Effects of in utero exposure to 4.7 T MR imaging conditions on fetal growth and testicular development in the mouse. Magn Reson Imaging 1996;14(3):263-274.
- 37. Narra VR, Howell RW, Goddu SM, Rao DV. Effects of a 1.5-Tesla static magnetic field on spermatogenesis and embryogenesis in mice. Invest Radiol 1996 Sep;31(9):586-590.
- 38. Fernie KJ, Reynolds SJ. The effects of electromagnetic fields from power lines on avian reproductive biology and physiology: a review. J Toxicol Environ Health B Crit Rev 2005;8(2):127-140.
- 39. Gye MC, Park CJ. Effect of electromagnetic field exposure on the reproductive system. Clin Exp Reprod Med 2012 Mar;39(1):1-9.
- 40. Kesari KK, Behari J. Evidence for mobile phone radiation exposure effects on reproductive pattern of male rats: role of ROS. Electromagn Biol Med 2012 Sep;31(3):213-222.
- 41. Saadeldin IM, Fadel AM, Hamada MM, El-Badry AA. Effects of exposure to 50 Hz, 1 Gauss magnetic field on reproductive traits in male albino rats. Acta Vet Brno 2011;80(1):107-111.
- 42. Ghanbari M, Mortazavi SB, Khavanin A, Khazaei M. Simultaneous effects of exposure to microwaves and noise on male rats’ sperm parameters and total antioxidant capacity. Health Scope 2013;1(4):179-184.
- 43. Mollasadeghi A, Mehrparvar AH, Atighechi S, Davari MH, Shokouh P, Mostaghaci M, et al. Sensorineural hearing loss after magnetic resonance imaging. Case reports in radiology 2013;2013:510258.
- 44. Swami CG, Ramanathan J, Charan Jeganath C. Noise exposure effect on testicular histology, morphology and on male steroidogenic hormone. Malays J Med Sci 2007 Jul;14(2):28-35.
- 45. Rajaei F, Farokhi M, Ghasemi N, Pahlevan AA. Effects of extremely low-frequency magnetic field on mouse epididymis and deferens ducts 2009;7(3):85-89.
- 46. Schaap K, Christopher-De Vries Y, Crozier S, De Vocht F, Kromhout H. Exposure to static and time-varying magnetic fields from working in the static magnetic stray fields of MRI scanners: a comprehensive survey in the Netherlands. Ann Occup Hyg 2014 Nov;58(9):1094-1110.
- 47. Schenck JF. Safety of strong, static magnetic fields. J Magn Reson Imaging 2000 Jul;12(1):2-19.