|
Abstract
Objectives: Responding to Pandemic Influenza A (H1N1) virus alert in 2009, Ministry of Health (MoH), Sultanate Of Oman arranged task force to deal with the emergency. MOH published articles in newspapers, prepared guidelines and hospitals were assigned to admit patients of H1N1. All the patients suspected of H1N1 were admitted and isolated as per the guidelines. This report describes clinical features and outcomes of 65 laboratory confirmed cases of H1N1 in Muscat, Sultanate of Oman.
Methods: From July to October 2009, 101 cases suspected of suffering from Pandemic Influenza A (H1N1) virus were admitted and isolated in Al Nahdha Hospital in Muscat. All the patients on admission were tested for H1N1, by real time reverse transcriptase polymerase chain reaction (RT-PCR). Immediately on admission, all of them were treated with Oseltamivir and antibiotics.
Results: Of the 65 confirmed cases of H1N1, 53.84% were males. Age of patients varied from 14 to 60 years, while 53.7% were aged between 31 to 55 years. Results showed that 70.8% had underlying co-morbidity; among which diabetes mellitus and respiratory illness were common. The most common presenting symptoms were fever (95%) and cough (94%). Also, 20% of the patients had leucopenia and 10.8% leucocytosis. Deranged LFT was observed in 26 (42.6%) of cases while 14 (21.5%) had hypokalemia. One patient (0.01%) with underlying severe co-morbidity died. Two patients (3.1%) had ARDS (Acute Respiratory Distress Syndrome); both recovered. Radiological infiltration was documented in 84.6% of cases, with lower zone involvement as the common finding. Hospital stay was between 1 to 12 days, 49.2% of patients were discharged within 3 days.
Conclusion: Mainly adult population was affected during this epidemic. H1N1 infection can lead to severe illness. Incidence of H1N1 was higher in patients with underlying co-morbidity. Timely intervention and administration of Oseltamivir may need to be modified.
Keywords: Pandemic Influenza A; H1N1; Muscat; Sultanate of Oman.
Introduction
In April 2009, USA government reported confirmed human cases of H1N1.1,2 From late March 2009, Mexican health authorities confirmed increasing number of ILI (Influenza like Illness) cases.3 The H1N1 virus isolated from affected cases had molecular features of North American and Eurasian swine, avian and human influenza virus,4-6 which was not previously detected in pigs or humans. There was also an increase number of the young population being affected as compared to very young or elderly people as is the case when seasonal influenza is concerned. In June 2009, WHO (World Health Organization) raised the pandemic level to 6 after documenting human to human transmission of the virus in at least three countries of two WHO regions.7
In response to the global pandemic alert, the Sultanate of Oman started reporting ILI cases along with laboratory confirmed cases of H1N1. This case study describes the clinical features and outcome of the first 65 laboratory confirmed cases of H1N1 admitted in the reference hospital in Sultanate of Oman.
Methods
The Department of Communicable Disease Surveillance and Control (DCDSC) Ministry of Health (MoH), Sultanate of Oman, in 2005 prepared a document on "National Pandemic Influenza Preparedness Plan" which was updated annually. Based on current available information on the novel H1N1 virus, some modifications were incorporated in 2009. A National guideline was published based on recommendations of World Health Organization (WHO) which was adapted from guidelines provided by the U.S. center for disease control. The objective was to have reference document for the concerned people in order to reduce the impact of pandemic on morbidity and mortality. With increasing cases of H1N1 globally, in May 2009, MoH formed a National Task force of National Spokesperson, National and Regional Rapid response team for Influenza H1N1.
Based on WHO guidelines, the following definitions were incorporated:
Suspected Case
Acute febrile respiratory illness (Fever >38ºC) with onset:
Within 7 days of close contact with a confirmed case of H1N1 influenza A virus OR
Within 7 days of travel to countries where one or more confirmed case of H1N1 influenza A virus were reported OR
Resides in a community where there were one or more confirmed cases of H1N1 influenza A virus
Probable case
Suspected case with an influenza test that is positive for influenza A, but is unsubtypable by reagents used to detect seasonal influenza virus infection OR
Suspected case who died of an unexplained acute respiratory illness and who is considered to be epidemiologically linked to another probable or confirmed case.
Confirmed case - Suspected or Probable case with laboratory confirmed H1N1 influenza A virus infection by one or more of the following tests:
Real- time RT- PCR
Viral culture
Four-fold rise in H1N1 influenza A virus specific neutralizing antibodies.
Close contact - Having cared for, lived with, or had direct contact with respiratory secretions OR body fluids of a probable or confirmed case of H1N1 influenza A virus.
All major newspapers published instructions from the MoH spokesperson with important telephone numbers in order to contain the H1N1 infection. Thermal scanners were installed at the main airport and surveillance of suspected cases carried out round the clock. Suspected cases were isolated and screened for H1N1. Al Nahdha Hospital in Muscat, apart from Regional Hospitals in the Sultanate of Oman, was designated as a reference hospital for admission of suspected cases of H1N1. In June 2009, Al Nahdha Hospital opened a new 20 bedded ward with all the required facilities in order to deal with the current pandemic situation. Admissions for the suspected cases of H1N1 started in July 2009. Data for the H1N1 positive patients admitted from July till October 2009 was analyzed. A proforma was prepared to collect all the available data of admitted patients. Confidentiality of patients was maintained by noting only Inpatient numbers. Collected data was reviewed by physicians.
As per the MoH guidelines adult (age: >13 years) patients having symptoms of Influenza like illness and opacity on chest radiographs were admitted. Whenever indicated, patients needing Intensive care (ICU) were transferred to nearby Royal Hospital, which is a tertiary care hospital in Muscat.
On admission, patients were isolated and underwent the following investigations before commencing treatment; complete blood count (CBC), Erythrocyte sedimentation rate (ESR), Random Blood Sugar (RBS), Renal Function (UE1), Liver Function Test (LFT), Creatinine Kinase (CK), C reactive protein (CRP), Lactic dehydrogenase (LDH), Arterial Blood Gas (ABG), Blood for bacterial culture, Chest X-Ray (CXR) and available sputum was sent for bacterial culture. Additional blood investigations were done as per the presenting clinical features of the patient. Patients with underlying risk factors, co-morbidity and presenting with hypoxia were admitted in high dependency area.
Treatment was started immediately on admission with Oseltamivir (75 mg bid) and relevant antibiotic as per the presenting features and guidelines of ATS CAP (American Thoracic Society, Community Acquired Pneumonia).8 In hospital, patients were closely followed till they were discharged as per the ATS CAP criteria.8 Patients were instructed to report to hospital immediately on relapse of symptoms. H1N1 patients who were discharged from Al Nahdha Hospital were followed within a week in out patient department stated specifically for these patients.
The collected data was analyzed by the hospital statistician after approval from the head of the department. Approval from the review board was not indicated in view of interest of public health.
For microbial studies, diagnostic specimens were collected at the time of admission to Hospital and before starting any medications. Special swabs with Polyester or Dacron tip were used. Two nasopharyngeal swabs and 2 throat swabs were collected. Samples kept in viral transport medium and stored at 2-8ºC.
Alternatively, nasopharyngeal aspirate or Bronchial wash samples collected in viral transport medium and stored at 2-8ºC. Samples were sent to The Central Public Health Laboratory. Real Time RT- PCR (Reverse Transcriptase polymerase chain reaction) testing was done in accordance with the published guidelines from WHO.9 Initially, results were available within two hours, which gradually increased up to one week due to overwhelming number of suspected H1N1 samples.
This study covered laboratory confirmed H1N1 positive cases admitted in Al Nahdha hospital only. All the data was analyzed using SPSS software 14.0 and using mainly descriptive statistical analysis method. Continuous variables were summarized as mean +/- SD. For categorical variables the percentage of patients in each category were calculated.
Results
All the patients admitted were mainly from Muscat region of Sultanate of Oman, which is a mixed population of Omanis and expatriates. We started receiving suspected cases of H1N1 for admission from July 2009. From July to October 2009, 101 patients suspected to be suffering from H1N1 were admitted in Al Nahdha Hospital. Out of which 65 cases were confirmed to be suffering from H1N1 by real time RT-PCR test. Data of these 65 laboratory confirmed cases of H1N1 were collected and analyzed. (Table 1)
The majority of patients were Omanis (74%). Amongst the patients, males constituted a larger proprtion (53.84%) compared to females. More than half of patients with H1N1 were between the age group of 31 to 55 years as compared to only 13.9% beyond 55 years of age. The time between onset of symptoms and admission to the hospital ranged from 1 to 14 days (average: 5.3 days). The results also showed that 46 (70.8%) patients had underlying pre-existing medical condition (Table 1). Diabetes Mellitus was found to be the most common followed by respiratory illness, consisting of Bronchial Asthma, COPD and Bronchiectasis. All the cardiac patients had underlying ischemic heart disease; two of them had undergone CABG (coronary artery bypass graft). Two patients of Renal Transplant and one of Multiple Myeloma were on immunosuppressive therapy while one HIV patient was on anti retroviral treatment.
Table 1: Characteristics of 65 cases with confirmed H1N1 infection.HCW= Health care workers
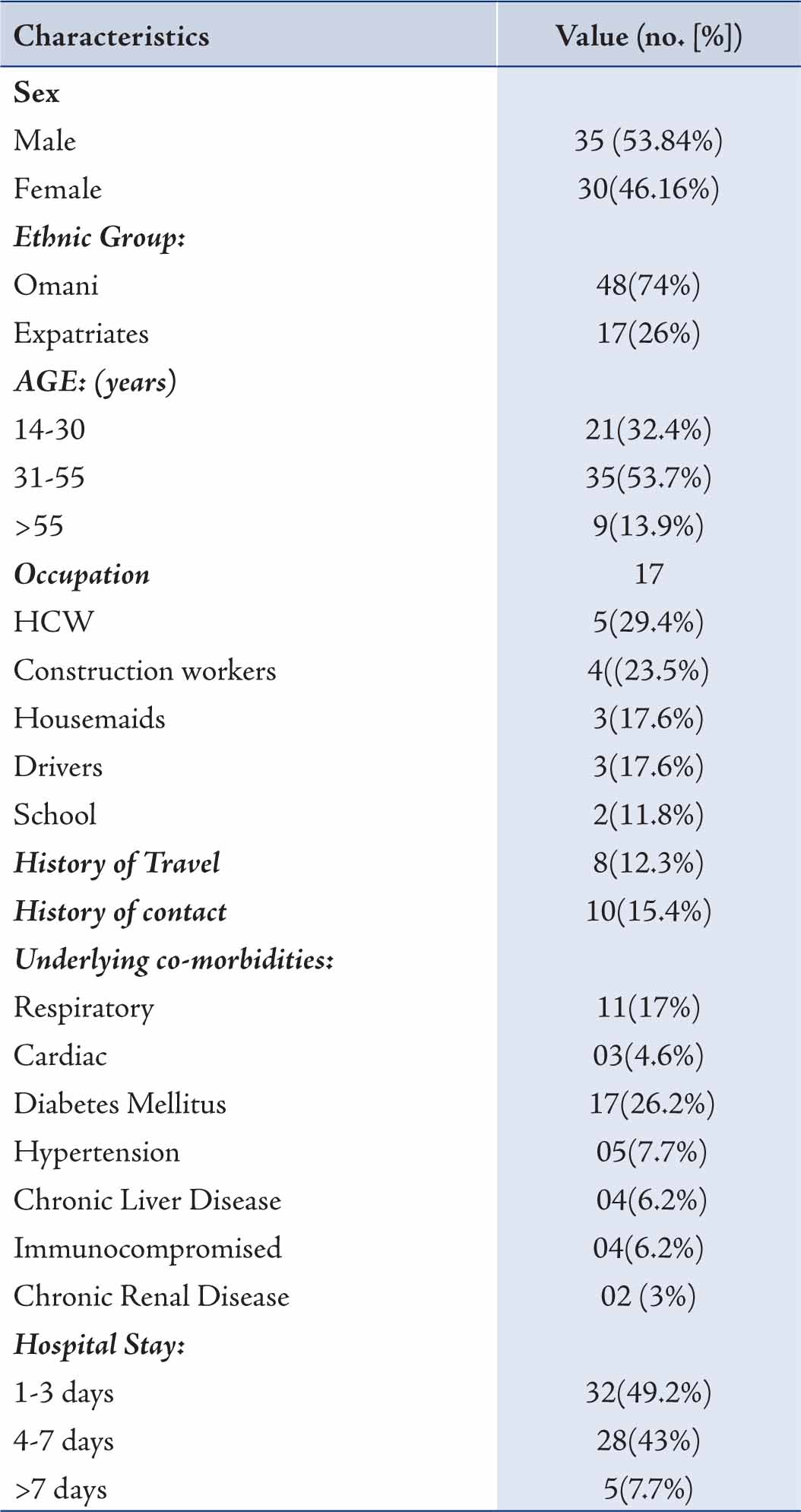
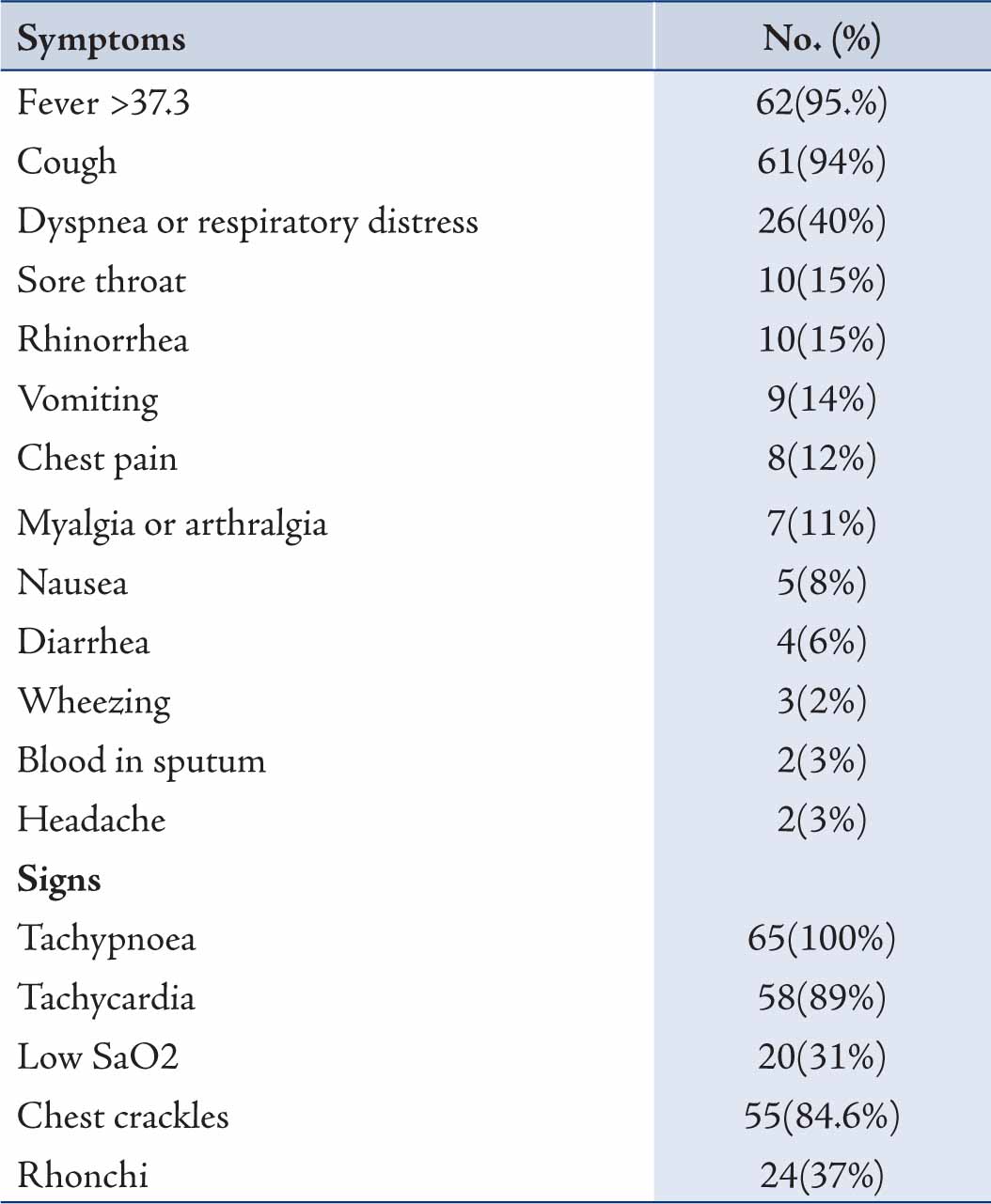
Chronic Renal Failure was found in two patients. Hepatitis B and C were found to be the cause of Chronic Liver disease. Fever (>37.3ºC) and cough were the commonest presentations (Table 2); while vomiting, nausea and diarrhea were the next common complaints. Only 2 patients complained of headaches and 31% of patients presented with hypoxia; of which 18 (27.7%) patients had ALI (Acute Lung Injury) and 2 (3.1%) had ARDS (Acute Respiratory Distress Syndrome). Patients in whom contact with H1N1 was documented, all of them were positive for H1N1. From the available records, no particular occupation was found to be common in the H1N1 patients.
Table 2: Clinical features of the admitted 65 H1N1 positive patients.
Laboratory and chest radiography results are shown in Tables 3 and 4. C reactive protein (CRP) was elevated in 96.2% patients followed by other inflammatory markers, CK and LDH. Very high CK and LDH (>1000) were found in 2 and 7 patients, respectively. Leucopenia was more common than leucocytosis, while lymphopenia occurred in only one patient. Sodium was normal in all patients, while hypokalemia was found in 21.5% patients. Lower zone involvement with patchy infiltration of 1 to 2 quadrants was a common radiological finding compared to extensive consolidation. (Table 3)
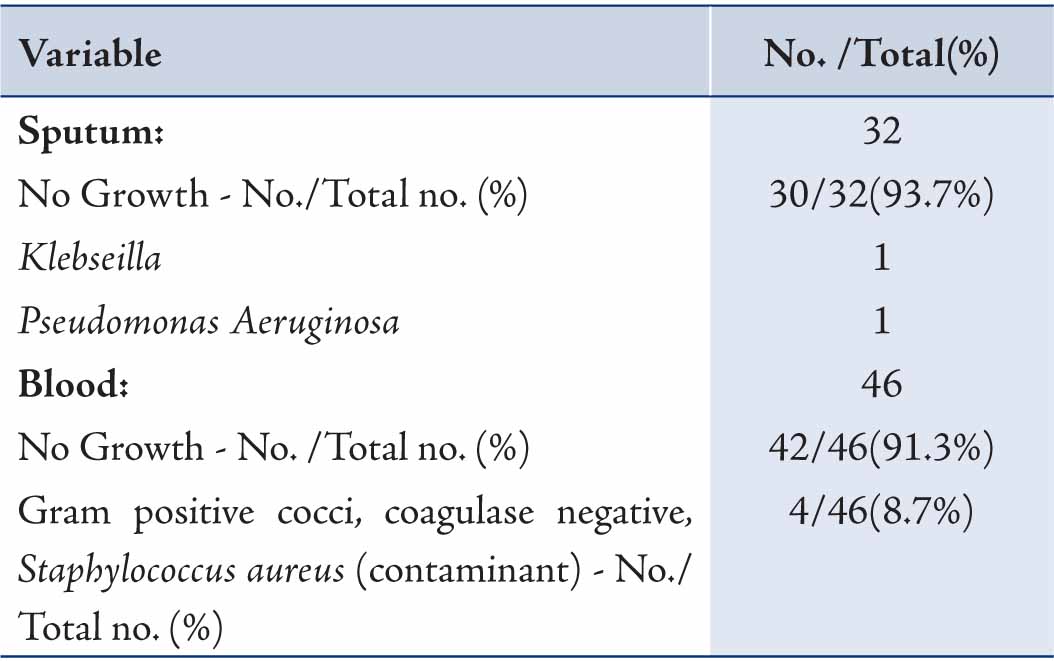
Of the samples collected for sputum culture, only two grew pathogenic bacteria. Similarly, almost all of the blood cultures were sterile; while the remaining confirmed to be contaminant after discussion with the microbiologist. (Table 4)
All the patients received Oseltamivir and antibiotics on admission irrespective of their earlier exposure to the same. The duration of Oseltamivir was 5 days in the majority of patients (95.4%). Antibiotics were continued for 7 days in all patients, and most of the patients were discharged within a week of admission to Hospital; however, seven patients had to stay back due to persistence of symptoms.
Table 3: At admission, details of laboratory investigations and Chest X-ray findings of H1N1 patients.
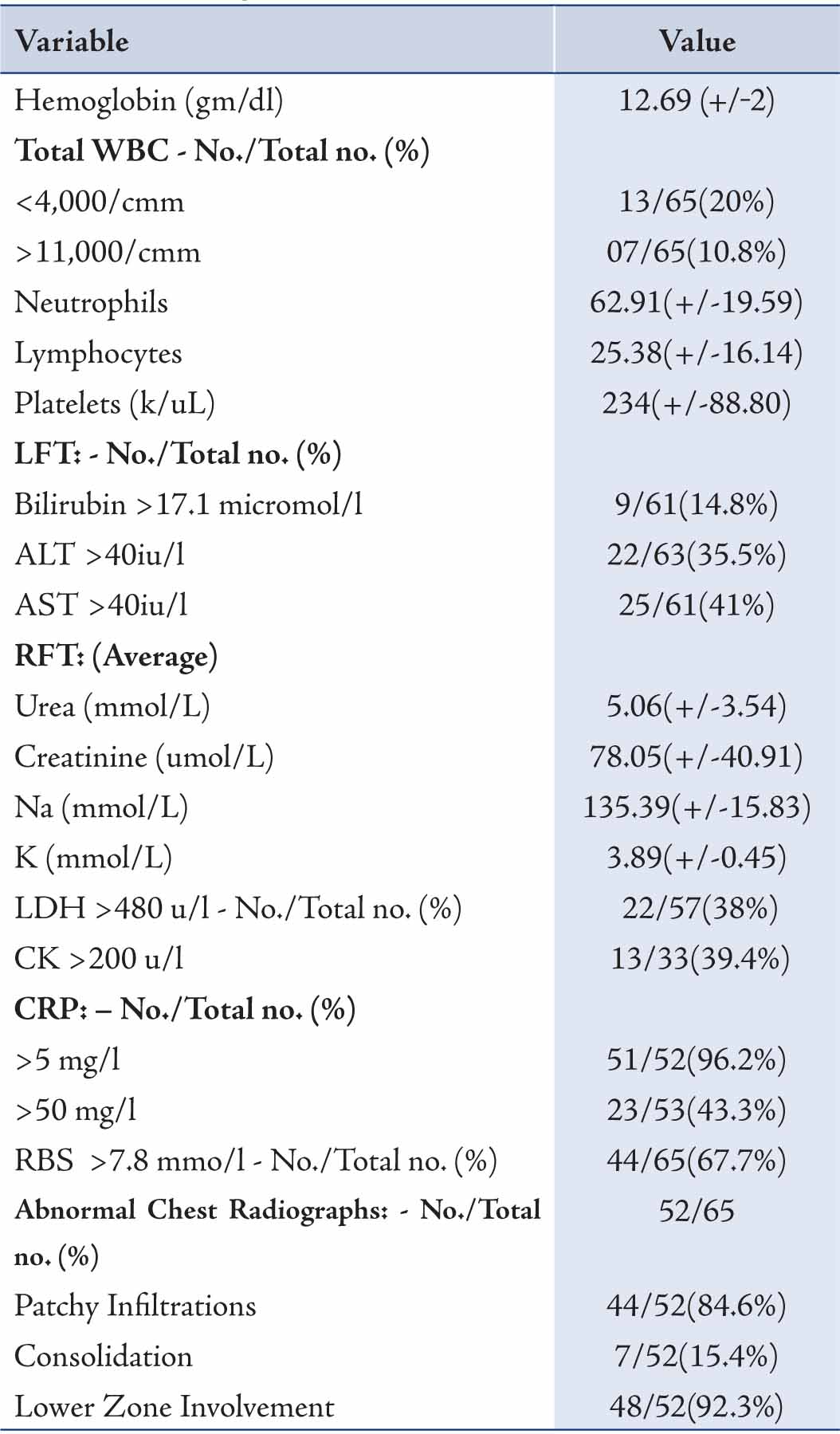
RBS = Random Blood Sugar; WBC = White blood cells. +/- = means +/- standard deviation.
Table 4: Sputum and blood culture findings of H1N1 positive patients.
One elderly patient died due to ventricular fibrillation, having underlying Diabetes Mellitus and chronic ischemic heart disease. Two patients were transferred to Royal Hospital for ICU (Intensive Care Unit) managements in view of ARDS (Acute Respiratory Distress Syndrome); both patients had successful outcomes and were discharged home. All other patients were followed up within a week of discharge, and were all free of symptoms. None of them returned after their first follow up in the clinic and were presumed to be doing well. Nausea was the commonest complaint (20%) after the administration with Oseltamivir. Vomiting was reported in 7 cases; while diarrhea developed in 3 patients after commencing treatment. Furthermore, mild rash developed in 2 patients. However, all the adverse events responded to symptomatic treatment.
Discussion
The present pandemic H1N1 influenza contains a combination of gene segments from swine, avian and human lineages.10 Although humans have previously been exposed to influenza A (H1N1) subtype, the present virus has caused sustain human to human transmission. This strain was different from earlier three episodes of global pandemic in 1918, 1957 and 1968 which were known as Spanish flu (H1N1), Asian flu (H2N2) and the Hong Kong flu (H3N2). Predominant morbidity and mortality in the former was among young and middle aged persons, while it was among infants and elderly people in the Asian and Hong Kong flu.
We are reporting 65 laboratory confirmed H1N1 cases that were admitted and isolated at the reference hospital in Muscat, Sultanate of Oman from July 2009 to October 2009. The adult population was mainly affected in our study in contrast to seasonal influenza hospitalization when admissions were for extremes of age.11 The incidence of H1N1 was low in the elderly which could be comparable to the incidence reported in a USA study.12 Clinical presentations of H1N1 admitted were generally similar to the earlier reported cases of seasonal influenza.13 Most of the patients in our study presented with fever and cough, which is similar to the patients in the USA,14 Japan,15 and in Mexico.16 The reported incidence of nausea, vomiting, diarrhea, was 39% in the USA study but was much lower in our patients.12 Diabetes and Respiratory illness constituted major co-morbidity in our patients as compared to a Chinese study,17 where it was only 0.7% and 1.9%, respectively; whereas underlying respiratory illness was common (36%) in USA patients.12
Very few bacteria could be detected from sputum and blood culture collected at the time of admission. This could be due to the difficulty in establishing specific bacterial diagnosis among patients with bacterial co infection by routine laboratory tests used. The incidence of Leucopenia was found in 20% of our cases which was similar to the cases found in China.17 CRP was high in almost all the cases in our study. Other laboratory findings included elevated LDH and CK, which could be due to associated myositis, which were also reported in the Mexican study.18 The presence of Hypokalemia in our patients was almost similar to 25.4% of patients in the Chinese study.17 While the incidence of Hypokalemia was much lower compared to 43-90% of hospitalized cases with SARS (Severe Acute Respiratory Syndrome).19,20 The mechanism of hypokalemia however, is unknown.
Most of our patients had chest radio graphical abnormality involving lower zones. Patchy infiltrates affecting one or two quadrants of the lung was more common compared with three or four in Mexican patients.18 In order to correlate radiological findings with underlying cause, better studies are needed especially during influenza outbreaks. During 1957-58 influenza outbreaks, Louria et al. reported findings of diffuse bilateral infiltration in patients with primary influenza viral pneumonia, whereas lobar infiltrates were seen in patients with secondary bacterial infections.21
Patients found to be hypoxemic had an average oxygen saturation of 86%. At the beginning, all of them were oxygenated with simple oxygen mask. Non responders required re-breathing masks. Two of them later deteriorated and remained hypoxic even with non re-breathing mask and had to be transferred to tertiary care hospital. These patients were successfully ventilated and later discharged home. Low incidence of ARDS could result from early presentation and minimal lung involvement in our cases, compared to extensive lung involvement and median oxygen saturation of 71% in the Mexican patients.18
As reported most of the lung damage was primarily due to infection with influenza virus.18 The possible mechanism of damage could be due to direct injury to the respiratory epithelium with a secondary cytokine storm.22 Co-infection with other respiratory virus could also explain the increase in pathogenicity.23,24
All the studied patients received Oseltamivir,25 and antibiotics on admission. Bacterial cultures in sputum or blood did not show significant growth. Recent guidelines of IDSA recommend the use of antiviral drugs in patients who need hospitalization following seasonal influenza.26 Even though recommendation of commencing antiviral therapy within 48 hours is the strongest, other studies have reported a reduction in mortality of hospitalized patients, even when such therapy was initiated 48 hours after the onset of illness.27 Though bacteria are considered to be common in patients presenting with CAP (community acquired pneumonia); influenza can be considered in differential diagnosis. It would be worthwhile to consider antiviral treatment in these patients; particularly in patients with underlying illness. Thus, early antiviral treatment is beneficial and it should not be withheld for patients who present late. Future studies should be undertaken locally to determine the incidence of CAP caused by viruses.
This study was not without limitations. For instance; prior seasonal vaccination information was not available in spite of the program being in place. Also, no other viral studies were carried out for these patients and all were considered to be suffering from H1N1 only. Furthermore, no follow up real time RT-PCR was done to confirm virus shedding, and not all information was collected for all patients in spite of common data collection form.
Conclusion
Overall, this study describes the first presentation of H1N1 cases admitted in Oman. The clinical features and outcome of the patients were comparable to other studies. In most cases, the patients who were admitted were young. Although the risk factors for acquiring H1N1 are still unknown; the incidence of H1N1 was highest amongst patients with underlying co-morbidities in the current study. One elderly patient died due to ventricular fibrillation, with severe underlying multiple co-morbidities. The low incidence of ARDS could be explained by early presentation and minimal lung involvement. Nevertheless, it would be worthwhile to investigate the efficacy of the present seasonal influenza vaccine to prevent H1N1 outbreak at some point in the future.
Acknowledgements
We thank the administrators of Al Nahdha Hospital, the Ministry of Health to permit us to conduct this study. We also thank Mr. Abdul Rahim for statistical analysis and all the H1N1 team at Al Nahdha Hospital, Muscat, Sultanate of Oman for managing these patients. The authors reported no conflict of interest and no funding was received for this work. |