|
Introduction
Metabolic syndrome (MS) is characterized by the variable coexistence of excess body fat, hyperinsulinemia (insulin resistance and glucose intolerance), dyslipidemia (high triglycerides and total cholesterol plasma levels), and hypertension.1,2 The presence of metabolic syndrome predicts a two-to-four-fold increase in the risk of cardiovascular disease and death,3 and the risk of developing type 2 diabetes is increased five-to-nine-fold.4
Insulin stimulates glucose uptake into tissues, and its ability to do so varies greatly among individuals. Resistance to the action of insulin leads to insulin resistant syndrome. Hyperinsulinemia results to prevent loss of glucose tolerance in insulin resistant individuals. The combination of insulin resistance and compensatory hyperinsulinemia predispose to the development of a cluster of abnormalities, including some degree of glucose intolerance, an increase in plasma triglycerides and a decrease in HDL-cholesterol concentrations. The cluster of changes associated with insulin resistance has been said to comprise syndrome X (metabolic syndrome).5
The MS pathogenesis is multifactorial and is related to central obesity, a sedentary lifestyle, an unbalanced diet and genetic predisposition. Insulin resistance is described as the central feature of MS.6 The renin-angiotensin system (RAS) is an important link between MS and cardiovascular diseases. All of the main RAS components are present in adipose tissue.7 RAS consists primarily of an enzymatic cascade in which angiotensinogen (AGT) is converted to angiotensin I (Ang I), and subsequently to Ang II by the actions of renin and angiotensin converting enzyme (ACE), respectively.8
Increased levels of Ang II have been observed in both obesity and diabetes patients. RAS components, especially AGT found in adipose tissue are closely related to the Ang II effects on insulin resistance.9,10 Furthermore, AGT secretion, as well as Ang II formation in adipocytes are increased in MS patients promoting adipocyte growth, which could explain the positive correlation between high blood pressure and increased adipose-tissue mass in these patients.11
Treatment of the MS encompasses two goals. The first is to address its underlying causes, namely obesity. The second goal is to treat all of its component clinical risk factors.1,12 As metabolic syndrome involves a clusters of a number of risk factors including hypertension, dyslipidemia, abdominal obesity, and hyperglycemia; it is therefore in patients with MS, an effective antihypertensive agent with minimal, if any, negative effects on metabolic parameters should be used.13
The patients in the present study are hypertensive patients having markers of metabolic syndrome. Thus, the aim of the present study is to investigate the effects of two antihypertensive drugs losartan (Ang II receptor blocker) and enalapril (ACE inhibitor) on BP and other markers of MS.
Methods
One hundred and twenty six newly diagnosed hypertensive, patients with other markers of metabolic syndrome participated in this study. They were selected from the out-patient clinic in Ibn-Sina teaching hospital in Mosul city. The study protocol was approved by regional Research Ethics Committees at the College of Medicine and Mosul Health Administration. The study was an open, controlled, comparative, clinical trial of two months duration, performed during the period between 1st December 2007 and 1st June 2009.
The patients were divided into two main groups: 1) Losartan group: consisted of 60 patients, with an age range between 39 and 68 years. They were kept on losartan (Angizar 50 mg, Micro pharmaceutical industries, Co. Ltd., India) for 2 months on 50 mg once daily oral dose; and 2) enalapril group: consisted of 66 patients, with an age range of 35 to 68 years. They were kept on enalapril (Enalapril 20 mg, Asia pharmaceutical industries, Co. Ltd., Aleppo-Syria), for 2 months on a 20 mg once daily oral dose.
The inclusion criteria included hypertensive patients (stage I) who meet the diagnostic criteria of metabolic syndrome according to the American National Cholesterol Education Program-Adult Treatment Panel III,2 blood pressure greater than or equal to 130/ 85, waist circumference greater than 102 cm for men and 88 for women, fasting blood glucose levels ≥110 mg/dl (≥6.1 mmol/l), triglycerides ≥150 mg/dl (3.9 mmol/l), and HDL-cholesterol <40 mg/dl (1.04 mmol/l) for men and <50 mg/dl (1.3 mmol/l) for women. Whereas, the exclusion criteria included patients having a current diagnosis of type 1 or type 2 diabetes mellitus, patients on antihypertensive therapy or any drug that affects blood pressure, patients with a history of hepatic, cardiac, renal or blood diseases or other diseases which may interfere with the study, patients having hypersensitivity to angiotensin converting enzyme inhibitors or angiotensin II receptor antagonists, patients with a history of severe or resistant hypertension, patients with secondary hypertension of any etiology, pregnant or breast feeding women, and patients with serious disorders which may limit the ability to evaluate the efficacy of the trial drugs.
Seventy apparently healthy, non-obese, normotensive individuals, whose age ranged between 40 and 62 years, were used as a control group. The selection of each control should have the BMI within normal range (18-25 kg/m2).
Waist circumference in (cm) was determined with a standard tape measure, as the point midway between the costal margin and iliac crest in the mid-axillary line, with the subject standing and breathing normally.14 Blood pressure was measured for each subject by the usual mercury sphygmomanometer from the arm in the sitting position. Measurement was taken after at least five minutes of rest.
Serum glucose concentration (FSG) was estimated by glucose-oxidase-peroxidase colorimetric method,15 using a kit supplied by BIOCON (Germany). Serum triglycerides and HDL-Cholesterol were measured using special Kits supplied by BIOLABO (France).
The effects of the drugs on the markers of MS were assessed by comparing the results of each drug before and after drug administration.
Statistical analysis was performed using the unpaired t-test to compare between the ages of the two groups, and between the baselines data of the patient and control group. Paired student t-test was used to compare the results before and after drug therapy. The statistical results were considered significant at p=0.05 or less.
Results
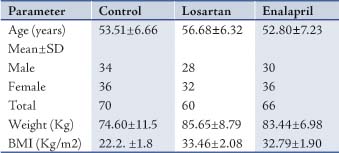
The age, weight, BMI and number of males and females of the patients and controls are presented in Table 1. There were no significant differences between the age of participants in the 3 groups (p>0.5). However, there were significant differences between weight and BMI in the patient groups and the control group, (p<0.001).
Table 1: Characterestics of patients and controls.
Baseline measurement of waist circumference, BP, FSG, and triglycerides of patient groups showed a significant elevation compared with the control group (p<0.001), while HDL-cholesterol showed lowered values compared with the controls (p<0.001). (Table 2)
Table 2: Baseline data of the patient groups as compared with the control group (Mean±SD).
SBP= Systolic blood pressure; DBP= Diastolic blood pressure; FSG= Fasting serum glucose; HDL= High density lipoproteins.
Comparison of the markers of MS before and after treatment with losartan, or enalapril showed a significant reduction of all markers except for triglycerides, while HDL-cholesterol showed a significant elevation only in the losartan group, (Tables 3, 4). On the other hand, there was a significant reduction in BMI and weight in patients after therapy administered with either losartan or enalapril. (Table 5)
Table 3: Markers of metabolic syndrome before and after treatment with losartan (Mean±SD).
Table 4: Markers of metabolic syndrome before and after treatment with enalapril (Mean±SD).
Table 5: Effects of losartan and enalapril on body weight and BMI of the patients.
Discussion
The present study demonstrates a beneficial effect of losartan or enalapril on BP and other markers of MS. A significant reduction of measures of obesity (weight, BMI and WC) were obtained after therapy with losartan or enalapril. Obesity may play a central role in the development of MS, thus increasing obesity is positively correlated with blood pressure, fasting glucose, and triglycerides; and negatively correlated with changes in HDL cholesterol.16 Reports in the literature demonstrate a relationship between renin angiotensin aldosterone system (RAAS) and obesity; and a significant increase in the activity and concentrations of (RAAS) components have been reported in obese subjects.17-19 Engeli et al.20 reported that weight reduction is associated with a reduction of angiotensinogen levels, renin, aldosterone and ACE activity, as well as the reduction of angiotensinogen levels, which was highly correlated with a decline in waist circumference. In the present study, both enalapril and losartan significantly reduced body weight, BMI, and WC in patients with MS. Enalapril is an ACE inhibitor and losartan is an AII receptor antagonist, they act by reducing the activity of RAAS in the body. Thus, their effects may be related to reducing the activity and the effect of ACE system on obesity.
A significant reduction of BP was obtained after 2 months treatment with both drugs, indicating beneficial effects of these drugs in the treatment of BP in patients with MS. Although both drug classes are highly effective in lowering BP in patients with essential hypertension,21-23 their comparative antihypertensive effectiveness are uncertain in patients with metabolic syndrome.24 The association between BMI and BP have been demonstrated,25 and there is evidence to suggest that obesity is a casual factor in the development of hypertension in obese individuals.26 Accordingly, the beneficial effect of losartan and enalapril on BP reported in the present study may be related mostly to their effects on RAAS, and partly on body weight, BMI and WC.
The present study demonstrates the beneficial effects of losartan and enalapril on FSG. The patients in the present study were hypertensive; having FSG concentration of ≥110 mg/dl. ACE inhibitors and Ang IIR antagonists may exert beneficial effects on glycemic control through a variety of mechanisms related to the inhibition of the angiotensin II, which activates the sympathetic nervous system resulting in the impairment of insulin secretion and peripheral glucose uptake. Angiotensin II also impairs pancreatic blood flow and enhances insulin resistance.27 The findings from recent clinical trials support the hypothesis that suppression of RAAS, either by inhibition of ACE or blockade of the AngT1R, substantially lowers the risk of type 2 diabetes.28-31
Regarding the effects of losartan and enalapril therapy on triglycerides and HDL-cholesterol; there were no significant effects observed on triglycerides after treatment with losartan or enalapril, and a significant elevation of HDL-Cholesterol was obtained only after therapy with losartan. The effect of losartan on HDL-cholesterol was in agreement with other reports, which also revealed beneficial effects of losartan on HDL-cholesterol levels.32,33
Conclusion
Losartan and enalapril can reduce the markers of MS and may be regarded as useful antihypertensive agents for the treatment of hypertensive patients having markers of metabolic syndrome.
Acknowledgements
The authors reported no conflict of interest and no funding was received on this work.
References
1. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004 Jan;109(3):433-438.
2. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001 May;285(19):2486-2497.
3. Ford ES, Giles WH. A comparison of the prevalence of the metabolic syndrome using two proposed definitions. Diabetes Care 2003 Mar;26(3):575-581.
4. Bindler RC, Massey LK, Shultz JA. Metabolic syndrome in a multi-ethnic sample of school children: Implications for the pediatric nurse. J Pede Nurse 2007;22:43-58 .
5. Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev 1995 Jul;75(3):473-486.
6. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2003 Feb;163(4):427-436.
7. Strazzullo P, Galletti F. Impact of the renin-angiotensin system on lipid and carbohydrate metabolism. Curr Opin Nephrol Hypertens 2004 May;13(3):325-332.
8. Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, et al. The adipose –tissue rennin-angiotensin- aldosterone system: role in the metabolic syndrome. Biochem Cell Biol 2003;35:807-825 .
9. Giacchetti G, Sechi LA, Rilli S, Carey RM. The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab 2005 Apr;16(3):120-126.
10. Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol 2004 Oct;287(4):R943-R949.
11. Prasad A, Quyyumi AA. Renin-angiotensin system and angiotensin receptor blockers in the metabolic syndrome. Circulation 2004 Sep;110(11):1507-1512.
12. Deedwania PC, Gupta R. Management issues in the metabolic syndrome. J Assoc Physicians India 2006 Oct;54:797-810.
13. Siegel D, Swislocki AL. Effects of antihypertensives on glucose metabolism. Metab Syndr Relat Disord 2007 Sep;5(3):211-219.
14. Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol 1994 Mar;73(7):460-468.
15. Lotta JA, Turner K. Evaluation of trinder’s glucose oxidase method for measuring glucose in serum and urine. Clin Chem 1975;2:1754-1760.
16. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005 Oct;112(17):2735-2752.
17. Cooper R, McFarlane-Anderson N, Bennett FI, Wilks R, Puras A, Tewksbury D, et al. ACE, angiotensinogen and obesity: a potential pathway leading to hypertension. J Hum Hypertens 1997 Feb;11(2):107-111.
18. Engeli S, Sharma AM. Emerging concepts in the pathophysiology and treatment of obesity-associated hypertension. Curr Opin Cardiol 2002 Jul;17(4):355-359.
19. Umemura S, Nyui N, Tamura K, Hibi K, Yamaguchi S, Nakamaru M, et al. Plasma angiotensinogen concentrations in obese patients. Am J Hypertens 1997 Jun;10(6):629-633.
20. Engeli S, Böhnke J, Gorzelniak K, Janke J, Schling P, Bader M, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension 2005 Mar;45(3):356-362.
21. Townsend R, Haggert B, Liss C, Edelman JM. Efficacy and tolerability of losartan versus enalapril alone or in combination with hydrochlorothiazide in patients with essential hypertension. Clin Ther 1995 Sep-Oct;17(5):911-923.
22. Verdecchia P, Schillaci G, Reboldi GP, Sacchi N, Bruni B, Benemio G, et, al. Long-term effects of losartan and enalapril, alone or with a diuretic, an ambulatory blood pressure and cardiac performance in hypertension: a case-control study. Blood Press Monit 2000;5:167-193 .
23. Fagard R, Lijnen P, Pardaens K, Thijs L, Vinck W. A randomised, placebo-controlled, double-blind, crossover study of losartan and enalapril in patients with essential hypertension. J Hum Hypertens 2001 Mar;15(3):161-167.
24. Karagiannis A, Mikhailidis DP, Athyros VG, Kakafika AI, Tziomalos K, Liberopoulos EN, et al. The role of renin-angiotensin system inhibition in the treatment of hypertension in metabolic syndrome: are all the angiotensin receptor blockers equal? Expert Opin Ther Targets 2007 Feb;11(2):191-205.
25. Tesfaye F, Nawi NG, Van Minh H, Byass P, Berhane Y, Bonita R, et al. Association between body mass index and blood pressure across three populations in Africa and Asia. J Hum Hypertens 2007;21:5-7 .
26. Sharma AM, Pischon T, Engeli S. Scholze. Choice of drug treatment for obesity-related hypertension: where is the evidence? Hypertens 2001;19:667-674 .
27. Jandeleit-Dahm KA, Tikellis C, Reid CM, Johnston CI, Cooper ME. Why blockade of the renin-angiotensin system reduces the incidence of new-onset diabetes. J Hypertens 2005 Mar;23(3):463-473.
28. Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet 1999 Feb;353(9153):611-616.
29. Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002 Mar;359(9311):995-1003.
30. Yusuf S, Gerstein H, Hoogwerf B, Pogue J, Bosch J, Wolffenbuttel BH, et al; HOPE Study Investigators. Ramipril and the development of diabetes. JAMA 2001 Oct;286(15):1882-1885.
31. Henriksen EJ, Jacob S, Kinnick TR, Teachey MK, Krekler M. Selective angiotensin II receptor receptor antagonism reduces insulin resistance in obese Zucker rats. Hypertension 2001 Oct;38(4):884-890.
32. Koh KK, Han SH, Chung WJ, Ahn JY, Jin DK, Kim HS, et al. Comparison of effects of losartan, irbesartan, and candesartan on flow-mediated brachial artery dilation and on inflammatory and thrombolytic markers in patients with systemic hypertension. Am J Cardiol 2004 Jun;93(11):1432-1435, A10.
33. Kyvelou SM, Vyssoulis GP, Karpanou EA, Adamopoulos DN, Zervoudaki AI, Pietri PG, et al. Effects of antihypertensive treatment with angiotensin II receptor blockers on lipid profile: an open multi-drug comparison trial. Hellenic J Cardiol 2006 Jan-Feb;47(1):21-28.
|