Ovarian cancer is a common gynecological cancer in women. It is characterized by having a poor prognosis with a five-year survival rate of less than 35%. The majority of cases are diagnosed at an advanced stage.1 In Oman, ovarian cancer is the seventh most common cancer in women.2 The Ministry of Health reported in its Cancer Incidence Registry (2011) a crude incidence of 1.8 and age-standardized incidence rate of 3.1 per 100,000, taking into consideration the estimated mid-year Omani population in 2011 of 2,137,807 with sex ratio of 983 females per 1000 males.2
Different guidelines are available for the management of different cancers including ovarian cancer. The National Institute for Health and Clinical Excellence (NICE) guidelines on the recognition and initial management of ovarian cancer addresses the issue of screening symptomatic women presenting to primary care.3 Important factors for the early diagnosis of ovarian cancer need health providers and women to be made aware of this disease and primary care physicians to initiate investigations early. Thus, it is crucial to assess certain risk factors for women who present with pelvic masses by optimizing health policies and providing sensitive and specific biomarkers for detecting the disease early; hence, providing early surgical staging procedures followed by appropriate treatment in specialized reference centers helping to stop overburdening these centers with benign manageable conditions.
Currently, the standard tools for detecting ovarian cancer are pelvic ultrasonography and measuring serum cancer antigen 125 (also called carbohydrate antigen 125; CA-125) levels, which could be combined with the menopausal status to calculate the risk malignancy index (RMI) and is considered a simple and affordable test.4 However, due to the performance limitations of the standard tools and aims to improve the sensitivity, specificity, and positive predictive value of tumor markers in ovarian cancer, a number of new biomarkers have been studied and evaluated to be used in combination with CA-125. Of these, human epididymis protein 4 (HE4), was identified as a promising marker.5 HE4, also called whey-acidic-protein (WAP) and four-disulfide core domain protein 2 (WFDC2), was originally described as an epididymis-specific protein that belongs to a four-disulfide core family. This comprises a heterogeneous group of small acid- and heat-stable proteins of divergent function.6 It is highly over-expressed in epithelial ovarian cancers (EOC) compared to normal ovarian epithelium.7–9
Moore et al10 developed a new numerical score to predict the risk of ovarian malignancy called the Risk of Ovarian Malignancy Algorithm (ROMA), which incorporates the results of HE4, CA-125, and menopausal status. ROMA was studied by many investigators and found to be a promising biomarker for predicting ovarian cancer.11 HE4 together with CA-125 can improve the accuracy of ovarian cancer detection. Additionally, HE4 is considered a valuable biomarker for discriminating ovarian cancer from ovarian endometriosis making it a more specific marker than CA-125.12
The aim of this study was to evaluate the validity of CA-125 as a currently used tumor marker for ovarian cancer and HE4 as a new biomarker for ovarian cancer in a pilot of patients presenting with ovarian lesions during their preoperative workup investigations. We then compared the performance of the four parameters, CA-125, HE4, RMI, and ROMA to determine the best marker to discriminate between benign and malignant ovarian tumors and the appropriate cut-offs of these markers.
Methods
This prospective, cross-sectional study was done at the Royal Hospital, Muscat, using a sample of 213 patients who attended the gynecology department between 1 March 2014 and 30 April 2015 for the evaluation of an ovarian mass. All patients were examined and assessed using pelvic ultrasonography by specialized gynecologists. Blood specimens from these patients were obtained during their first assessment for laboratory work up. All cases underwent surgical intervention at a later stage to obtain a histopathological diagnosis, which was used as the gold standard test. All clinical and laboratory data were collected using the hospital information system AL Shifa 3 Plus.
The blood samples of the patients were collected during their first assessment, before surgical intervention, using standard serum separator tubes (SST) for different biochemical profiles including tumor markers. The samples were centrifuged immediately after collection to get the sera and then analyzed. The remaining sera were stored at -20 oC. After collecting the required number of specimens, serum HE4 was measured.
Both CA-125 and HE4 assays were done by a two-step immunoassay using the Architect i2000 SR Immunoassay Analyzer (Abbott Laboratories, Illinois, US), which uses chemiluminescence microparticle immunoassay technology. All manufacturer recommendation for maintenance, calibration, and internal quality assessment were followed for both assays. The between run precisions of CA-125 were 2.8%, 3.2%, and 2.2% for the levels of internal quality control (low, middle, and high concentration of CA-125) materials, respectively. In-house analytical verification of HE4 was performed before it was adopted, as it was a newly introduced test in the Royal Hospital. Within- and between-run imprecision studies for HE4 assay were done by running three levels of internal quality control five times in the same run and five times on five different days. The within-run precisions of the three levels of internal quality control for the HE4 assay were 1.9%, 2.8%, and 2.2% whereas the between-run precisions were 1.5%, 4.8%, and 5.7%, respectively.
Patients were grouped according to age (pre- and postmenopausal) and lesion type (benign or malignant). The postmenopausal status was defined as one year or more of amenorrhea or an age of 50 years or more if the woman had undergone a hysterectomy. From the variables collected, the RMI was calculated using the formula: RMI 2 = U × M × serum CA-125
where U is the total ultrasound score, M is the menopausal status and CA-125 value in U/mL.13
ROMA was calculated using CA-125 and HE4 results as per the manufacturer’s recommendations (Abbott ARCHITECT ci8200; Abbott Laboratories, Illinois, US). This was followed as recommended by Moore et al, by calculating a predictive index (PI) for premenopausal and postmenopausal patients separately using equation 1 and 2 as follows:10
1. PI for premenopausal women: PI = -12.0 + 2.38*lnHE4 + 0.0626*ln(CA-125)
2. PI for premenopausal women: PI = -8.09 + 1.04*lnHE4 + 0.732*ln(CA-125)
The ROMA score was then obtained using the equation: ROMA % = exp PI / (1 + exp PI) × 100% where Exp PI = e PI
The cut-off value for CA-125 was 35 U/mL as recommended by the manufacturer and the cut-off value for RMI was 200 as proposed by Jacobs et al.14 The cut-off value for HE4 was 70 pmol/L, and for ROMA for high-risk premenopausal and postmenopausal women was 13.1% and 27.7%, respectively.10
A comparison study was done for the four parameters (CA-125, RMI, HE4, and ROMA) and the validity indicators including sensitivity, specificity, positive and negative predictive values (PPV and NPV) and efficiency were calculated. Both the receiver operating characteristic (ROC) curve and area under the curve (AUC) were calculated, and the most valid cut-offs were determined accordingly. For all statistical comparisons, a p-value < 0.050 was accepted as statistically significant. All statistical analysis was done using SPSS Statistics (SPSS Statistics, Chicago, US) version 22.
Results
This prospective study included 213 women who attended the gynecology clinic at the Royal Hospital, Muscat. One-hundred and sixty-two women (76%) were premenopausal, of whom 21 (13%) had a malignant ovarian lesion. Fifty-one (24%) women were postmenopausal, and 27 (53%) of these had malignant ovarian lesion [Table 1].
The total number of ovarian specimens was 213, of which 165 (77.5%) were benign, and 48 (22.5%) were malignant tumors. Table 2 shows the histopathology results of all ovarian specimens in pre- and postmenopausal cases. The histopathology classifications of ovarian tumors included surface epithelial-stromal, sex cord stromal, and germ cell tumors. Lesions that did not fit into one of these three groups were termed “others”.
Table 1: Patients’ demographic characteristics. Data are presented as mean±SD and median (range) unless otherwise indicated.
|
n (%) |
165 (78) |
48 (23) |
|
141 (87) |
21 (13) |
|
24 (47) |
27 (53) |
|
|
Age, years |
35±14
33 (13–80) |
50±18
55 (21–83) |
0.001 |
31±9
31 (13–50) |
32±8
33 (21–49) |
0.374 |
61±10
59 (47–80) |
64±9
64 (51–83) |
0.595 |
|
BMI,
kg/m2 |
28±6
27 (15–48) |
27±7
27 (15–45) |
0.374 |
27±6
27 (15–44) |
28±7
28 (15–49) |
0.767 |
30±6
29 (22–48) |
26±6
23 (19–39) |
0.463 |
B: benign; M: malignant; Null: nulliparity; Primi: primi-parity (1 child); Multi: multi-parity (>1 child).
Table 2: Histopathological types of ovarian tumors in the study population.
|
Epithelial |
Serous cystadenoma |
17 |
12 |
5 |
Serous adenocarcinoma |
20 |
5 |
15 |
| |
Mucinous cystadenoma |
10 |
8 |
2 |
Mucinous adenocarcinoma |
1 |
1 |
0 |
| |
Endometrial cysts |
34 |
34 |
0 |
Endometrial adenocarcinoma |
3 |
1 |
2 |
| |
Sermo-mucinous |
1 |
1 |
0 |
Undifferentiated |
1 |
0 |
1 |
| |
|
|
|
|
Borderline epithelial |
7 |
6 |
1 |
| |
Total, n (%) |
62 (37.6) |
55 (38.7) |
7 (29.2) |
Total, n (%) |
32 (66.7) |
13 (62.0) |
19 (70.4) |
|
Sex cord |
Fibroma |
4 |
3 |
1 |
Granulosa |
5 |
4 |
1 |
| |
Thecoma |
3 |
2 |
1 |
|
|
|
|
| |
Total, n (%) |
7 (4.2) |
5 (3.5) |
2 (8.3) |
Total, n (%) |
5 (10.4) |
4 (19.0) |
1 (3.7) |
|
Germ cell |
Teratoma |
33 |
29 |
4 |
Yolk sac cancer |
1 |
1 |
0 |
| |
Struma ovarri |
2 |
2 |
0 |
Immature teratoma |
2 |
2 |
0 |
| |
Total, n (%) |
35 (21.2) |
31 (21.8) |
4 (16.7) |
Total, n (%) |
3 (6.3) |
3 (14.3) |
0 (0.0) |
|
Others |
Simple cyst |
9 |
5 |
4 |
Secondaries |
7 |
1 |
6 |
| |
Functional cyst |
26 |
23 |
3 |
Lymphoma |
1 |
0 |
1 |
| |
Abscess |
7 |
5 |
3 |
|
|
|
|
| |
Para-ovarian cyst |
4 |
4 |
0 |
|
|
|
|
| |
Fibroid |
9 |
8 |
1 |
|
|
|
|
| |
Normal |
6 |
5 |
1 |
|
|
|
|
Pre: pre-menopausal; Post: post-menopausal.
The four variables (CA-125, RMI, HE4, and ROMA) were tested in the study by detailed descriptive analysis within the two main groups of benign and malignant lesions [Table 3]. In this setting, to test for a significant difference, non-parametric t-tests were applied (Kruskal-Wallis test and Mann-Whitney U test). All four parameters showed significantly higher median values within the malignant group when compared to the benign group. The distribution of the four variables was also checked through the different histopathology lesions. All showed a significant difference (p < 0.050) between benign and malignant groups except for the sex cord tumors in which the four tested variables were not statistically different between the lesion types.
Using the proposed cut-offs for the four tested variables, the validity indicators for the four parameters including their sensitivity, specificity, NPV, PPV, efficiency, and AUC are shown in
Table 4.
Table 3: CA-125, RMI, HE4, and ROMA values in all, pre-menopausal (pre) and post-menopausal (post) patient groups at their standard cut-offs. Data presented as mean ± SD and median (range).
|
CA-125,
U/mL |
62±132
23(1–978) |
1039±2326
261 (7–14507) |
67±141
24 (4–2296) |
927±3153
65 (7–14507) |
31±40
20 (5–199) |
1125±1454
458 (8–5733) |
|
RMI |
189±427
45 (4–3184) |
10751±19543
1777 (7–91728) |
164 ±374
40 (4–2296) |
3640±12631
260 (7–58028) |
336±651
140 (20–3184) |
15961±22332
7264 (128–91728) |
|
HE4,
pmol/L |
65.8±210.8
43 (18–2677) |
688.8±1122.4
207 (27–5932) |
42.3±43.8
41.8 (18–537) |
387.8±1280.8
66.9 (27–5932) |
180.3±537.8
51.0 (24–2677) |
923.0±941.2
7264 (128–91728) |
The p-value is < 0.001 for all variables (CA-125, RMI, HE4, and ROMA) between benign and malignant tumor in all, pre- and postmenopausal women.
Table 4: Validity indicators of the tested parameters in all, pre-menopausal (pre) and post-menopausal (post) patient groups at their standard cut-offs.
|
Sensitivity |
All |
79 |
77 |
71 |
75 |
| |
Pre |
67 |
57 |
57 |
52 |
| |
Post |
89 |
93 |
93 |
93 |
|
Specificity |
All |
62 |
82 |
90 |
88 |
| |
Pre |
60 |
85 |
93 |
90 |
| |
Post |
79 |
67 |
75 |
78 |
|
NPV |
All |
91 |
93 |
91 |
92 |
| |
Pre |
92 |
93 |
94 |
93 |
| |
Post |
86 |
89 |
90 |
90 |
|
PPV |
All |
38 |
56 |
68 |
65 |
| |
Pre |
20 |
36 |
55 |
44 |
| |
Post |
83 |
76 |
81 |
83 |
|
Efficiency |
All |
71 |
80 |
81 |
82 |
| |
Pre |
63 |
71 |
75 |
71 |
| |
Post |
84 |
80 |
84 |
85 |
|
AUC |
All |
0.809 |
0.853 |
0.824 |
0.837 |
| |
Pre |
0.673 |
0.724 |
0.674 |
0.680 |
Table 5: Comparison of the tested four parameters among patients with endometriosis and other benign ovarian lesions. Data are presented as mean ± SD and median (range).
|
CA-125,
U/mL |
133±197 |
43±102 |
<0.001 |
| |
64 (9–973) |
19 (1–978) |
|
|
RMI |
327±557 |
153±381 |
<0.001 |
| |
111 (26–2296) |
36 (4–3184) |
|
|
HE4, pmol/L |
43.6±14.1 |
71.6±236.3 |
0.845 |
| |
41.1 (21–78) |
42.8 (18–2677) |
|
|
ROMA, % |
6.9±5.6 |
9.4±14.4 |
0.462 |
Out of the 48 ovarian cancer cases, CA-125 detected 38 cases while HE4 detected 34. The four parameters were able to detect the various types of ovarian cancer except for sex cord/granulosa tumors in which these tools detected one out of five cases. The four parameters detected most epithelial tumors except for borderline lesions. CA-125 was able to detect four out of seven cases, and HE4 was able to detect two of seven cases only. The validity indicators for the four variables were also tested in EOC lesions alone and were compared to all cases of ovarian cancers. The highest calculated sensitivity was for CA-125 (88% in EOC vs. 79% in all) followed by RMI (84% in EOC vs. 77% in all) and ROMA (84% in EOC vs. 75% in all). HE4 measured the least sensitivity (78% in EOC vs. 71% in all).
In contrast, the false positive rates in different benign lesions were similar in most lesions except for endometriosis, teratoma, and fibroid lesions in which CA-125 level was raised in 27/34, 7/33, and 6/9 of cases respectively, compared to the HE4 level that was raised only in 3/34, 0/33 and 3/9 of cases, respectively. Two cases of fibroid lesions had Chronic Kidney Disease (CKD) stage 5 and one fibroid case had ascites, which are known to contribute to the false positive results.15,16
To compare endometriosis with other benign ovarian lesions, the medians of the four parameters were calculated in both groups as shown in Table 5. Both HE4 and ROMA showed no significant difference between the two types of lesions whereas for CA-125 and RMI the medians revealed significantly higher levels in the endometriosis lesion group.
Figure 1 shows the ROC curve of the four parameters in all, premenopausal, and postmenopausal groups. RMI showed a slightly higher AUC than the other parameters in all (0.853) and the premenopausal (0.724) women. However, all tested parameters were slightly better than CA-125 in these groups. ROMA and RMI had a slightly higher AUC in the postmenopausal group (0.944 and 0.941, respectively) and the AUC of HE4 was the lowest in this group (0.897). Using the ROC curve, different cut-offs were investigated to determine the optimal cut-off to get the appropriate sensitivity and specificity [Table 6].
Table 6: Sensitivity and specificity of the four parameters for premenopausal and postmenopausal patients at the standard cut-offs and optimal identified cut-offs.
|
CA-125, U/mL |
All |
35 |
79 |
62 |
82 |
71 |
86 |
| |
Pre |
35 |
67 |
60 |
60 |
57 |
78 |
| |
Post |
35 |
89 |
79 |
71 |
89 |
96 |
|
RMI |
All |
200 |
77 |
82 |
348 |
73 |
90 |
| |
Pre |
200 |
57 |
85 |
240 |
57 |
88 |
| |
Post |
200 |
93 |
67 |
944 |
85 |
96 |
|
HE4, pmol/L |
All |
70 |
71 |
90 |
77.5 |
71 |
96 |
| |
Pre |
70 |
57 |
93 |
63.6 |
91 |
52 |
| |
Post |
70 |
93 |
75 |
137.9 |
89 |
92 |
|
ROMA, % |
All |
- |
75 |
88 |
22.7 |
71 |
96 |
| |
Pre |
13.1 |
52 |
90 |
16.4 |
52 |
94 |
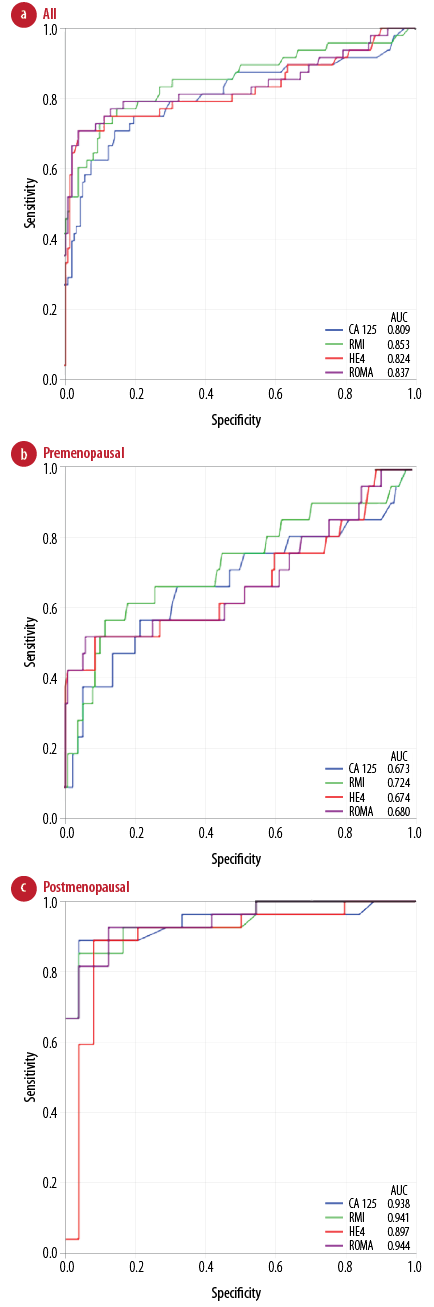
Figure 1: The receiver operating characteristic (ROC) curve and area under the curve (AUC) of CA-125, RMI, HE4, and ROMA for (a) all, (b) premenopausal, and (c) postmenopausal patients.
Discussion
CA-125 in this study had the highest sensitivity (79%) in the total study population and the premenopausal group (67%) compared to the other markers. However, in the postmenopausal group, CA-125 had a sensitivity of 89%, lower than the 93% sensitivity of the other three markers. In contrast, a high specificity of 90% was found for HE4 in the total study population. This was 93% in the premenopausal group and 75% in the postmenopausal group. CA-125 had the highest specificity of 79% in the postmenopausal group, compared to 78% for ROMA, 75% for HE4 and 67% for RMI. Hence, HE4, and ROMA showed a high specificity, and although they were less sensitive than CA-125 and RMI in premenopausal women, they were of comparable sensitivity in postmenopausal women in addition to their higher specificity.
All epithelial tumors were detected by one or more of the four markers except the borderline lesions. Of the seven cases with borderline epithelial lesions, CA-125 was the best marker for their detection. CA-125 detected four out of seven of cases, RMI and ROMA detected three out of seven of cases, and HE4 detected two of seven of cases. The four markers detected only one of five cases of sex cord/granulosa tumor.
Ferraro et al5 reported in their meta-analysis overlapped sensitivity of 79% for HE4 and CA-125, but a significantly higher specificity for HE4 (93%) compared to CA-125 (78%). Similarly, Anastasi et al12 reported a higher sensitivity for CA-125 (90%) compared to HE4 (87%) and a lower specificity for CA-125 (70%) compared to HE4 (100%) in the diagnosis of EOC. A prospective study by Richards et al17 noted that HE4 had a better specificity than CA-125 for the diagnosis of ovarian cancer in all women as well as in premenopausal women in addition to the higher ROC-AUC for HE4 compared to CA-125 in all women. ROMA was not inferior to the RMI calculation in their study population.
We reported that both CA-125 and HE4 were not sensitive to diagnose borderline ovarian cancer. To increase their sensitivity and specificity in this setting, it was suggested to perform serials of CA-125 and HE4 measurements along with ultrasound assessment.18 Also, CA-125 was reported as a poor marker for detecting granulosa cell tumors.19 When analyzing the false positive rates in different benign lesions, CA-125 was frequently elevated in patients with benign gynecological conditions particularly in premenopausal women compared to HE4 (38% vs. 10%, respectively). Some cases (mainly endometriosis) were found to have high levels of CA-125 and RMI compared to HE4 and ROMA. The medians of HE4 and ROMA values showed no significant difference between benign ovarian lesions and endometriosis, whereas the results of CA-125 and RMI revealed significantly higher levels in endometriosis. HE4 and ROMA can be useful markers when CA-125 levels are falsely elevated particularly in cases of endometriosis. Others also reported that measuring HE4 can be a valuable approach for distinguishing patients with ovarian endometrioma or other benign adnexal masses from those with ovarian malignancy, which may reduce other costs by reducing expensive diagnostic procedures.12 Similar to CA-125, HE4 values were noted to be falsely raised in the two cases with CKD stage 5 and one case with ascites, which has been previously reported for both markers.16
In this study, RMI appears to be comparable to ROMA, but a critical inspection may be needed in this setting since in our patient series the RMI score was calculated using an objective ultrasound assessment, which depends solely on the experience of the gynecologist. The calculated RMI value may be affected if ultrasound examination is performed by non-trained personnel including primary health care clinicians. Anton et al20 and Moore et al21 assessed the impact of using advanced computed tomography or magnetic resonance imaging on RMI score and its outcome. They found that the performance was not affected by these modifications, and no differences were noted in the accuracy of the four parameters for differentiating between the types of ovarian masses.
For CA-125 and RMI, we observed a significant increase in their specificity if the cut-off was increased to ≥ 60 U/mL for CA-125 and to ≥ 250 for RMI. For HE4, we noted an improvement in its specificity in the postmenopausal group when its cut-off was increased to 140 pmol/L. No significant change in the performance of ROMA was noted when its cut-off was altered for any groups. Winarto et al22 reported a better prediction of ovarian malignancy when using modified cut-offs for the different markers compared to the standard cut-offs, which resulted in higher specificity and accuracy but at the expense of reduced sensitivity. They reported that at modified cut-off values of CA-125 (165.2 U/mL), HE4 (103.4 pmol/L), RMI (368.7) and ROMA (28/54), the sensitivity and specificity was 67% and 75.4% for CA-125; 73.1% and 85.2% for HE4; 73.1% and 80.3% for RMI; and 77.6% and 86.9% for ROMA. When compared to the standard cut-off values, the sensitivity and specificity was 91% and 24.6% for CA-125; 83.6% and 65% for HE4; 80.6% and 65.6% for RMI; and 91.0% and 42.6% for ROMA.
Moszynski et al23 studied the usefulness of HE4 as a second-line test in the assessment of women with suspicious ovarian tumors. They concluded that HE4 had a higher specificity, accuracy, and positive predictive value than CA-125. However, the two markers are complementary and may be useful in situations when less experienced sonographers perform a pelvic ultrasound. This may suggest that HE4 is a more reliable test than RMI since the latter is dependent on ultrasound score. Therefore, taking into account the high sensitivity of CA-125 and high specificity of HE4, a panel of both tests using algorithms such as ROMA appears to be advantageous. Moore et al10,24 reported a sensitivity of 94.3% and specificity of 75% in one study and a sensitivity of 76.5% and specificity of 95% in another study when both were combined to differentiate benign from malignant ovarian lesions. The authors also reported a sensitivity of 93.8%, a specificity of 74.9% and a negative predictive value of 99% when using both markers in a ROMA.25 The combined panel has the advantage of being less likely to be elevated in benign tumors compared to CA-125, particularly in differentiating endometriosis from malignant ovarian tumors.
Recently a novel diagnostic index combining HE4, CA-125, and age was reported as a simple index that could be used to speed up the referral of women with suspected ovarian cancer and was independent of ultrasound and menopausal status.26 Additionally, there are genetic algorithms for risk assessment of ovarian cancer screening that have been recently described by applying classic genetic pedigree with a panel of biomarkers that identify both phenotypic and genotypic expression of high-risk markers followed by conventional and advanced ultrasonography. This approach might improve the screening process of asymptomatic high-risk women using this technology in specialized centers in the future.27 However, the inclusion of HE4 and use of algorithms in the workup investigations has to consider its cost-effectiveness and impact on the total budgetary expenditure of the overall service balanced by the additional advantages of its use, whether alone or in combination with CA-125 that allows calculation of the ROMA. This includes the number of patients’ referrals to gyne-oncology clinics as the available evidence still support CA-125 with lowered cut-off as a cost-effective strategy.28
Conclusion
Our study indicates that CA-125 and HE4, as well as ROMA and RMI values, are useful tools to differentiate between benign and malignant ovarian tumors. Although HE4 and ROMA were less sensitive than CA-125 and RMI in premenopausal women, they were of comparable sensitivity in postmenopausal women in addition to their higher specificity. HE4 and ROMA were more useful in distinguishing other benign ovarian tumors or endometriosis from ovarian cancer. HE4 can be a useful marker in situations where pelvic ultrasonography is performed by less experienced sonographers as in a primary care setting to triage further women presenting with adnexal lesions.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006 Nov;95(Suppl 1):S161-S192.
- 2. Cancer incidence in Oman, 2011, Dept of Non-Communicable Disease Surveillance and Control, Directorate General of Health Affairs, Ministry of Health, Oman: [Cited December 2015]. Available from: http///www.moh.gov.om.
- 3. NICE Clinical Guideline 122. The recognition and initial management of ovarian cancer. 2011. [Cited December 2015]. Available from: http://guidance.nice.org.uk/CG/Wave17/22.
- 4. Al-Musalhi K, Al-Kindi M, Ramadhan F, Al-Rawahi T, Al-Hatali K, Mula-Abed WA. Validity of Cancer Antigen-125 (CA-125) and Risk of Malignancy Index (RMI) in the Diagnosis of Ovarian Cancer. Oman Med J 2015 Nov;30(6):428-434.
- 5. Ferraro S, Braga F, Lanzoni M, Boracchi P, Biganzoli EM, Panteghini M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: a systematic review. J Clin Pathol 2013 Apr;66(4):273-281.
- 6. Kirchhoff C, Habben I, Ivell R, Krull N. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod 1991 Aug;45(2):350-357.
- 7. Hellström I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res 2003 Jul;63(13):3695-3700.
- 8. Huhtinen K, Suvitie P, Hiissa J, Junnila J, Huvila J, Kujari H, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer 2009 Apr;100(8):1315-1319.
- 9. Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res 2005 Mar;65(6):2162-2169.
- 10. Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2009 Jan;112(1):40-46.
- 11. Li F, Tie R, Chang K, Wang F, Deng S, Lu W, et al. Does risk for ovarian malignancy algorithm excel human epididymis protein 4 and CA125 in predicting epithelial ovarian cancer: a meta-analysis. BMC Cancer 2012;12:258.
- 12. Anastasi E, Granato T, Falzarano R, Storelli P, Ticino A, Frati L, et al. The use of HE4, CA125 and CA72-4 biomarkers for differential diagnosis between ovarian endometrioma and epithelial ovarian cancer. J Ovarian Res 2013;6(1):44.
- 13. Tingulstad S, Hagen B, Skjeldestad FE, Onsrud M, Kiserud T, Halvorsen T, et al. Evaluation of a risk of malignancy index based on serum CA125, ultrasound findings and menopausal status in the pre-operative diagnosis of pelvic masses. Br J Obstet Gynaecol 1996 Aug;103(8):826-831.
- 14. Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol 1990 Oct;97(10):922-929.
- 15. Arik N, Adam B, Akpolat T, Haşil K, Tabak S. Serum tumour markers in renal failure. Int Urol Nephrol 1996;28(4):601-604.
- 16. Nagy B Jr, Krasznai ZT, Balla H, Csobán M, Antal-Szalmás P, Hernádi Z, et al. Elevated human epididymis protein 4 concentrations in chronic kidney disease. Ann Clin Biochem 2012 Jul;49(Pt 4):377-380.
- 17. Richards A, Herbst U, Manalang J, Pather S, Saidi S, Tejada-Berges T, et al. HE4, CA125, the Risk of Malignancy Algorithm and the Risk of Malignancy Index and complex pelvic masses - a prospective comparison in the pre-operative evaluation of pelvic masses in an Australian population. Aust N Z J Obstet Gynaecol 2015 Oct;55(5):493-497.
- 18. Braicu EI, Van Gorp T, Nassir M, Richter R, Chekerov R, Gasimli K, et al. Preoperative HE4 and ROMA values do not improve the CA125 diagnostic value for borderline tumors of the ovary (BOT) - a study of the TOC Consortium. J Ovarian Res 2014;7:49.
- 19. Stine JE, Suri A, Gehrig PA, Chiu M, Erickson BK, Huh WK, et al. Pre-operative imaging with CA125 is a poor predictor for granulosa cell tumors. Gynecol Oncol 2013 Oct;131(1):59-62.
- 20. Anton C, Carvalho FM, Oliveira EI, Maciel GA, Baracat EC, Carvalho JP. A comparison of CA125, HE4, risk ovarian malignancy algorithm (ROMA), and risk malignancy index (RMI) for the classification of ovarian masses. Clinics (Sao Paulo) 2012;67(5):437-441.
- 21. Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller MC, Allard WJ, et al. Comparison of a novel multiple marker assay vs. the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol 2010;203(3):228.e1-6.
- 22. Winarto H, Laihad BJ, Nuranna L. Modification of cutoff values for HE4, CA125, the Risk of Malignancy Index, and the Risk of Malignancy Algorithm for ovarian cancer detection in Jakarta, Indonesia. Asian Pac J Cancer Prev 2014;15(5):1949-1953.
- 23. Moszynski R, Szubert S, Szpurek D, Michalak S, Krygowska J, Sajdak S. Usefulness of the HE4 biomarker as a second-line test in the assessment of suspicious ovarian tumors. Arch Gynecol Obstet 2013 Dec;288(6):1377-1383.
- 24. Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol 2008 Feb;108(2):402-408.
- 25. Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, et al. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol 2011 Aug;118(2 Pt 1):280-288.
- 26. Karlsen MA, Høgdall EV, Christensen IJ, Borgfeldt C, Kalapotharakos G, Zdrazilova-Dubska L, et al. A novel diagnostic index combining HE4, CA125 and age may improve triage of women with suspected ovarian cancer - An international multicenter study in women with an ovarian mass. Gynecol Oncol 2015 Sep;138(3):640-646.
- 27. Chu MM, Fishman D. Risk assessment for epithelial ovarian cancer: proposing a new approach to a deadly problem. Scand J Clin Lab Invest Suppl 2014;244:63-67, discussion 66-67.
- 28. Havrilesky LJ, Dinan M, Sfakianos GP, Curtis LH, Barnett JC, Van Gorp T, et al. Costs, effectiveness, and workload impact of management strategies for women with an adnexal mass. J Natl Cancer Inst 2015 Jan;107(1):322.